(UroToday.com) The 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) cancers symposium held in San Francisco, CA was host to a prostate cancer poster session. Dr. Stephen Freedland presented an EMBARK post hoc analysis of sexual activity-related patient reported outcome measures.
About 20 to 50% of patients with non-metastatic castration-sensitive prostate cancer (nmCSPC) experience biochemical recurrence (BCR) following primary definitive therapies. Continuous ADT has been the mainstay treatment in advanced prostate cancer; however, it is associated with significant adverse events, including loss of libido and erectile dysfunction.
EMBARK is a three-arm, randomized phase 3 trial that enrolled prostate cancer patients with high-risk biochemical recurrence, a PSA doubling time of ≤9 months, and a PSA ≥1 ng/ml after radical prostatectomy or ≥2 ng/ml above nadir following primary EBRT. Patients had no evidence of metastasis on conventional imaging. Patients were randomized to:
- Enzalutamide (160 mg) daily plus leuprolide every 12 weeks (n=355)
- Placebo plus leuprolide (n=358)
- Enzalutamide monotherapy (n=355; open label arm)
Enzalutamide plus leuprolide was demonstrated to have superior MFS outcomes compared to leuprolide alone (HR: 0.42, 95% CI: 0.30 – 0.61; p<0.001); similarly, enzalutamide monotherapy was also superior to leuprolide alone for MFS (HR: 0.63; 95% CI: 0.46 – 0.87; p=0.005).1 These results have recently led to enzalutamide becoming FDA approved for this indication in this patient population.
A prespecified patient reported outcome analysis previously showed that sexual activity (measured as a composite score using the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire-Prostate 25 [QLQ-PR251]) was better preserved with enzalutamide monotherapy versus leuprolide alone, with no significant differences between enzalutamide combination and leuprolide alone. This ad hoc analysis was performed to further assess the treatment effect on sexual activity in each comparison and enable shared decision-making between patients and physicians.

With regards to sexual activity item-level analysis, time to confirmed deterioration was defined as:
- Duration of time from the date of randomization to the date of the first deterioration in item rating of at least one point compared with the baseline score
- Confirmed at the next consecutive scheduled visit
- Measured by:
- QLQ-PR25 items 50 and 51
- Functional Assessment of Cancer Therapy-Prostate (FACT-P) items GS7, P5, and BL5.
- Pre-defined thresholds, confirmed at two consecutive visits
Patient reported outcomes were assessed at baseline and every 12 weeks thereafter until disease progression. The intent-to-treat population was analyzed. P-values were nominal and not adjusted for multiple comparisons.
Completion rates were excellent at ≥85%, except for the GS7 domain of the FACT-P questionnaire, due to the permissibility of a voluntary response (35 -36%). The time to confirmed deterioration was delayed in the enzalutamide mono versus leuprolide alone for QLQ-PR25 items 50 (interest) and 51 (activity), and FACT-P items GS7 (satisfaction) and BL5 (erectile function).

No significant differences were observed between enzalutamide combination and leuprolide alone, except for a shorter time to confirmed deterioration by a median of 0.06 months in BL5 (erectile function) with enzalutamide combination.
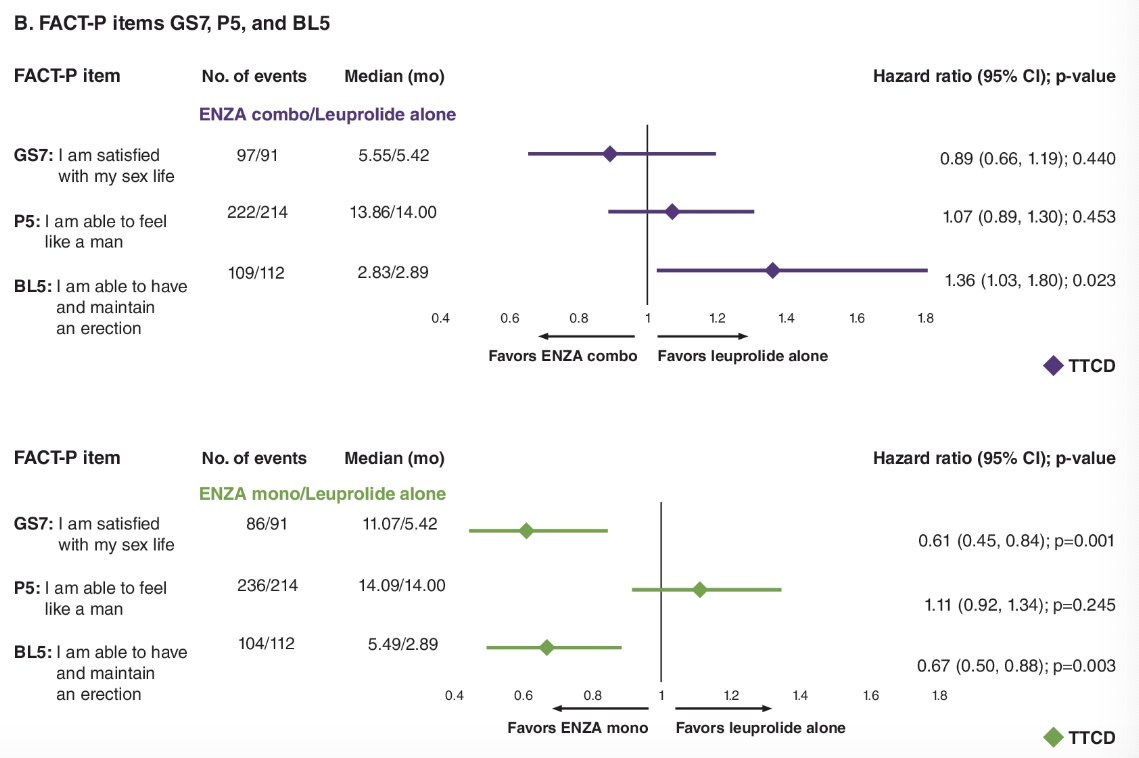
Dr. Freedland concluded that this ad hoc, item-level analysis of the EMBARK trial confirmed that sexual activity was better preserved with enzalutamide monotherapy, compared to leuprolide alone, in terms of interest, activity, satisfaction, and maintaining erection. Further, the sexual activity patient reported outcomes were similar between enzalutamide combination and leuprolide, implying there is no further sexual activity burden when adding enzalutamide to ADT.
Presented by: Stephen Freedland, MD, Professor, Department of Urology, Cedars Sinai Hospital, Los Angeles, CA
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, San Francisco, CA, January 25th – January 27th, 2024
References:


