(UroToday.com) The 2024 GU ASCO annual meeting featured a prostate cancer session and a presentation by Dr. Guilhem Roubaud discussing real-life data on [177Lu]Lu-PSMA-617 from the largest cohort in France. Previously, the VISION trial showed that [177Lu]Lu-PSMA-617 added to best standard of care prolonged imaging-based progression-free survival and overall survival in patients with PSMA-positive metastatic castration resistant prostate cancer (mCRPC).1 As such, the French Health Authorities granted a "cohort" early access on December 1, 2021, for [177Lu]Lu-PSMA-617 for this indication.
PSMA positive mCRPC patients pretreated with at least 1 taxane-based chemotherapy regimen and ≥1 androgen receptor pathway inhibitor were included in this real-world evidence study. [177Lu]Lu-PSMA-617 (7.4 GBq) was administered up to 6 cycles every 6 weeks. Patient characteristics and safety data were described for the entire population, and efficacy was analyzed within a sub-population with 6 months minimum follow-up after the first [177Lu]Lu-PSMA-617 injection.
Since December 1, 2021, 1,340 patients with mCRPC were included, and 696 patients were analyzed for efficacy: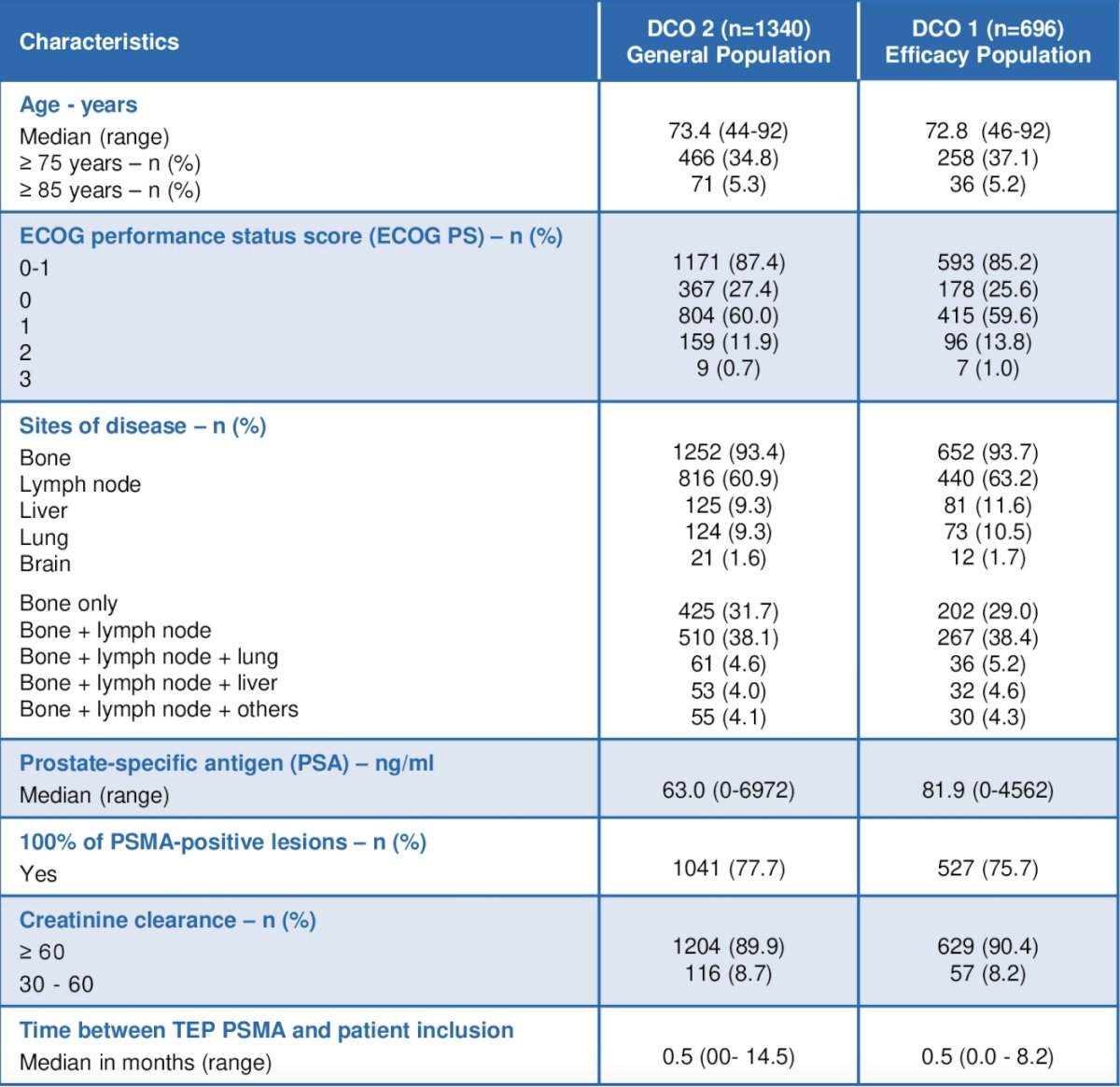
Compared to the VISION trial patients, those treated in the real world were older (73.4 vs 70.0 years), with a poorer ECOG performance status (0-1: 87.4% vs 92.6%) and a higher prevalence of lymph node metastasis (60.9% vs 49.7%). Furthermore, a higher proportion of patients received >= 2 novel hormonal agents and two taxanes:![[177Lu]Lu-PSMA-617 compared to VISION](/images/com-doc-importer/143-asco-gu-2024/asco-gu-2024-real-life-data-on-177lu-lu-psma-617-descriptive-analysis-on-the-largest-mcrpc-cohort-treated-in-france/image-1.jpg)
There were 1,085 patients that received a concomitant treatment, with almost all of them having received ADT. The distribution is shown in the following figure: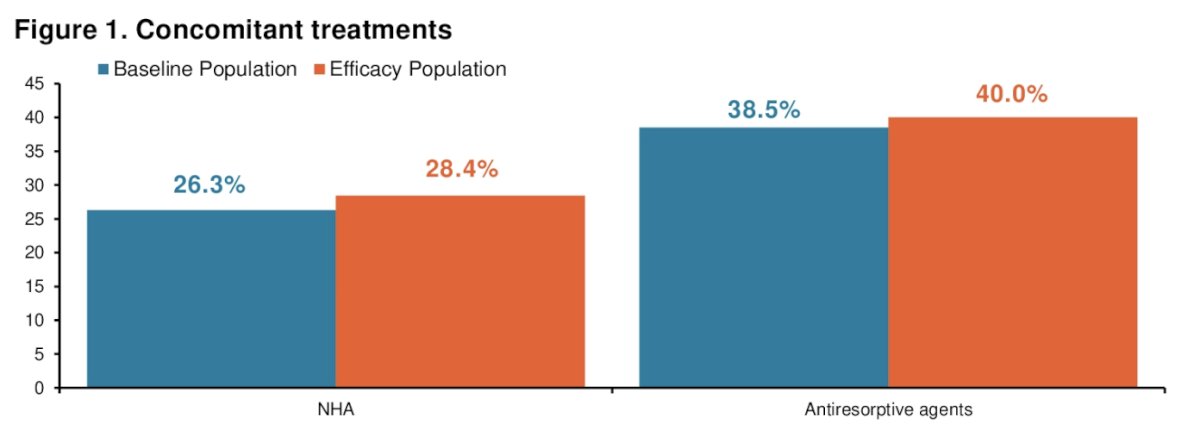
At the data cut-off of February 28, 2023, 99 patients were still under treatment, with a median of 4 cycles (5 cycles in VISION) received. Imaging follow-up was performed according to the investigator’s choice, in most cases this was both a CT and bone scan, and in some cases a PSMA-PET. Over a median follow-up of 6.7 months (range 6.2 – 6.7), the time to first PFS event was 7.3 months (95% CI 6.2-8.0). Overall, 68.4% patients had a decrease in PSA level at any time point, 12.7% had a stable PSA, and 18.0% had PSA progression. Most adverse events were as expected and no safety signal was identified despite a poorer conditioned patient at baseline. The frequent reported adverse events was hematologic toxicity (199/344):![[177Lu]Lu-PSMA-617 adverse events table](/images/com-doc-importer/143-asco-gu-2024/asco-gu-2024-real-life-data-on-177lu-lu-psma-617-descriptive-analysis-on-the-largest-mcrpc-cohort-treated-in-france/image-3.jpg)
Dr. Roubaud concluded his presentation discussing real-life data on [177Lu]Lu-PSMA-617 from the largest cohort in France with the following take-home points:
- Considering the population from the VISION trial, patients from the French early access program had poorer ECOG status, more metastasis, and were heavily pre-treated
- For those included until February 28, 2023, patients received a median of 4 cycles, with a median imaging-based PFS of 7.3 months
- Despite an altered general condition, safety data were similar to VISION, and no new safety signals were identified
- [177Lu]Lu-PSMA-617 tends to be used earlier in patients with mCRPC compared to the beginning of the French early assessment program
Presented by: Guilhem Roubaud, Department of Medical Oncology, Institut Bergonié, Bordeaux, France
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the Genitourinary (GU) American Society of Clinical Oncology (ASCO) Annual Meeting, San Francisco, CA, Thurs, Jan 25 – Sat, Jan 27, 2024.
References:


