(UroToday.com) The 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) cancers symposium held in San Francisco, CA was host to a prostate cancer trials in progress poster session. Dr. Luke Nordquist presented COMBAT, a study of 64Cu-SAR-BBN and 67Cu-SAR-BBN for the identification and treatment of GRPr-expressing metastatic castrate-resistant prostate cancer (mCRPC).
mCRPC is an advanced and lethal form of prostate cancer, with a median overall survival of ~3 and 2 years in clinical trial and real world settings, respectively. The prostate-specific membrane antigen (PSMA)-targeted agent 68Ga-PSMA-11 is currently approved for the selection of mCRPC patient candidates for treatment with 177Lu-PSMA-617. However, PSMA tumor expression is either absent or low in up to 10% of men with primary prostate cancer, 25% of men with biochemical recurrence, and 25% of men with mCRPC.1-4 As such, these patients are unlikely to benefit from PSMA-targeted treatment approaches and represent a significant unmet need for both imaging and therapy.
The Gastrin Releasing Peptide receptor (GRPr) is a transmembrane G-protein coupled receptor that has various physiological functions in the gastrointestinal tract and nervous system. This receptor protein is also upregulated in many human cancers, including prostate cancer.
A promising new theranostic pair, consisting of 64Cu-SAR-Bombesin (64Cu-SAR-BBN, imaging) and 67Cu-SAR-Bombesin (67Cu-SAR-BBN, therapy), targets the GRPr . This may offer a potential imaging and treatment option for patients with low or no PSMA expression. Translational data have demonstrated that 67Cu-SAR-BBN inhibits tumor growth and improves survival in a PC3 xenograft mouse model.3 These data provide the rationale for the COMBAT study, which aims to assess the safety and anti-tumour efficacy of 67Cu-SAR-BBN in mCRPC patients with GRPr-expressing disease
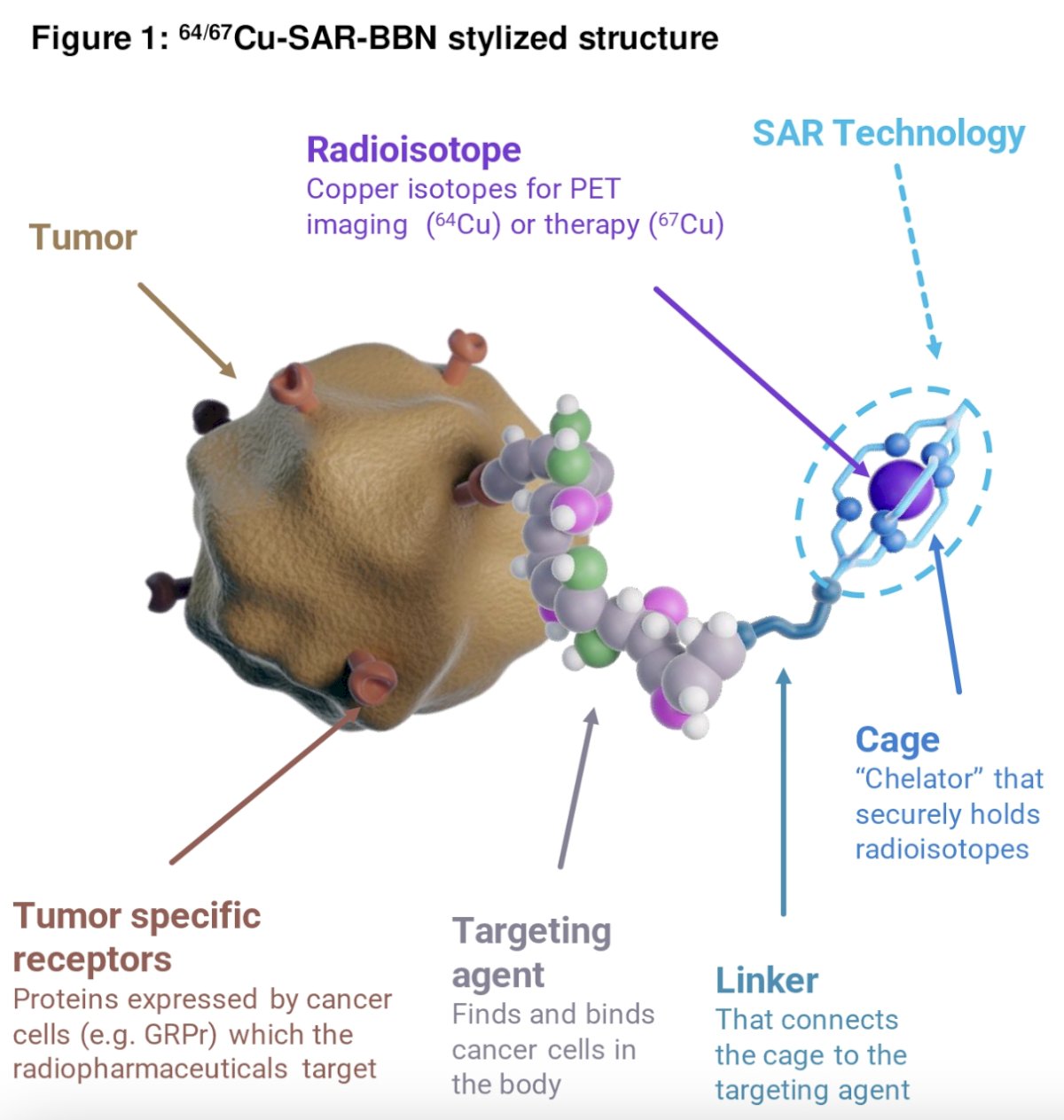
COMBAT is a multicenter, open-label, phase I/lla dose-escalation and cohort expansion study of 64Cu-SAR-BBN and 67Cu-SAR-BBN administered to patients with mCRPC meeting the following eligibility criteria:
- Life expectancy >6 months
- Histological, pathological, and/or cytological confirmation of prostate cancer
- Positive 64Cu-SAR-BBN PET/CT scan
- Castrate serum/plasma testosterone level (<50 ng/dL or <1.7 nmol/L)
- ≥1 metastatic lesion that is present at screening, CT, MRI, or bone scan imaging
- Adequate organ function and ECOG performance status of 0 – 2
- Have progressive mCRPC despite prior ADT and ≥1 ARPI. Progression based on ≥1 of the following: serum/plasma PSA progression, soft tissue progression, and/or progression of bone disease
- Ineligible for 177Lu-PSMA-617 therapy
- Previous treatment with a systemic radionuclide is allowed after a pre-specified washout period
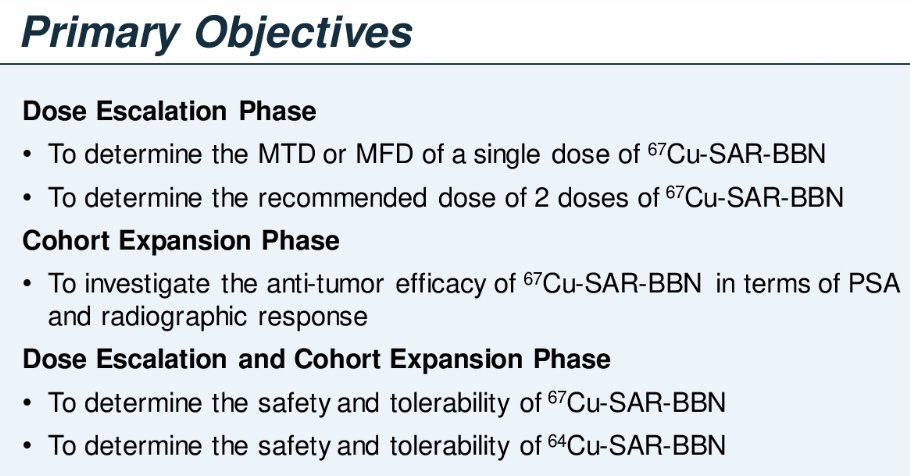
The primary study objectives are as follows:
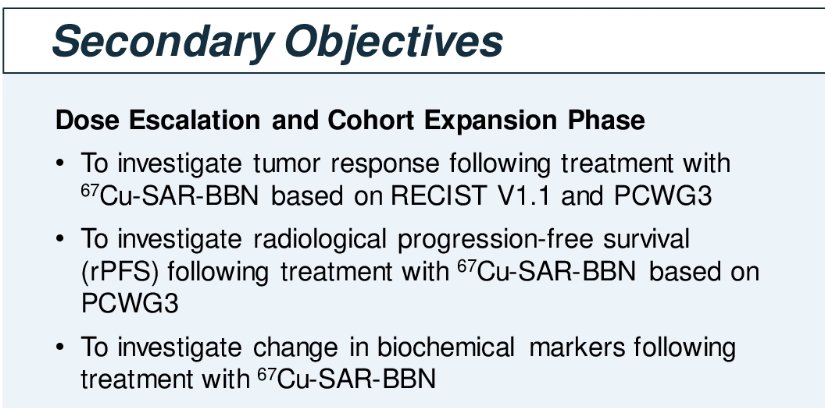
The secondary objectives include:
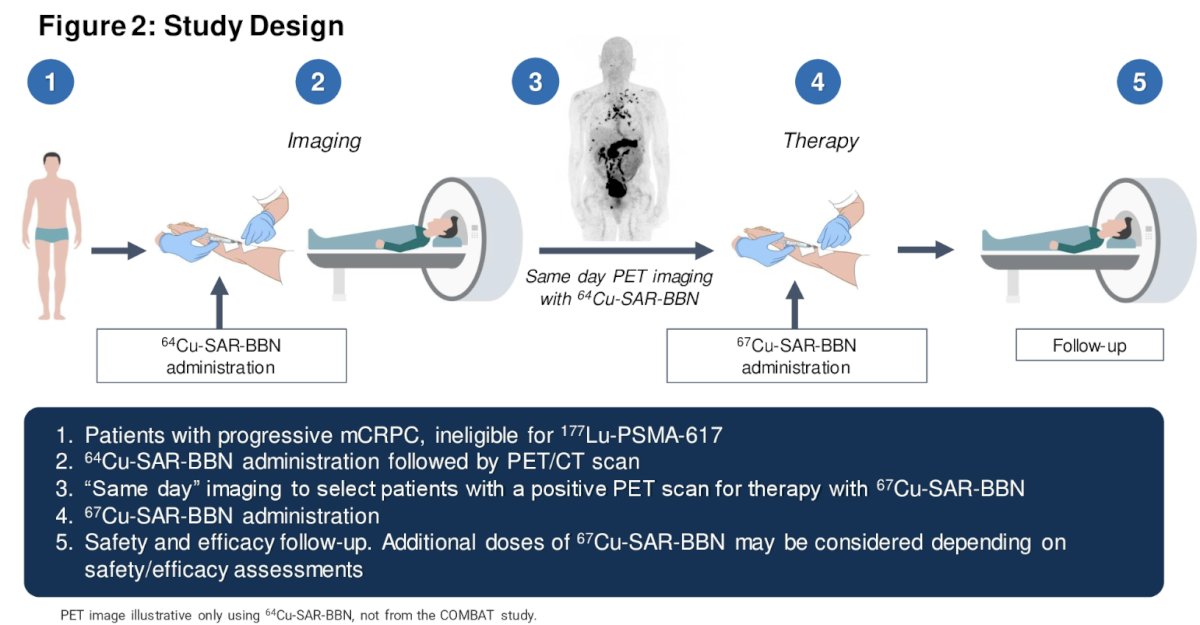
COMBAT is being conducted in two phases:
- Dose Escalation Phase (n=up to 24)
- Cohort Expansion Phase (n= 14)
The 67Cu-SAR-BBN dose levels investigated in the escalation phase include:
- Cohort 1: 6 GBq (single dose with dosimetry assessment)
- Cohort 2: 10 GBq (single dose)
- Cohort 3: 14 GBq (single dose)
- Cohort 4: Up to 28 GBq across two doses at MTD/MFD
- Additional doses may be administered during both phases of the study.

At the time of presentation, study enrolment for cohort 1 is currently underway at three active sites in the United States, with three additional sites in start-up.
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, San Francisco, CA, January 25th – January 27th, 2024
References:
- Afshar-Oromieh A, da Cunha ML, Wagner J, et al. Performance of [68Ga]Ga-PSMA-11 PET/CT in patients with recurrent prostate cancer after prostatectomy-a multi-centre evaluation of 2533 patients. Eur J Nucl Med Mol Imaging. 2021;48(9):2925-34.
- Vlachostergios PJ, Niaz MJ, Sun M, et al. Prostate-Specific Membrane Antigen Uptake and Survival in Metastatic Castration-Resistant Prostate Cancer. Front Oncol. 2021;11:630589.
- Huynh TT, van Dam EM, Sreekumar S, et al. Copper-67-Labeled Bombesin Peptide for Targeted Radionuclide Therapy of Prostate Cancer. Pharmaceuticals (Basel). 2022;15(6):728.


