(UroToday.com) The 2024 GU ASCO annual meeting featured a prostate cancer session and a presentation by Avina Rami discussing the impact of race on outcomes to 177-Lu-PSMA-617 (LuPSMA) for metastatic castration resistant prostate cancer (mCRPC). African American patients are more likely to develop and die from metastatic prostate cancer than non-Hispanic white patients.
Prior work suggests that African American and other minority patients derive equal benefit from taxane chemotherapy and androgen receptor pathway inhibitors for mCRPC as white patients. Previously, the VISION trial showed that LuPSMA had an overall survival benefit in mCRPC patients after prior taxane and androgen receptor pathway inhibitor.1 However, there are no data on racial differences in outcomes to LuPSMA.
This study queried an IRB-approved registry of all patients treated with at least two cycles of LuPSMA between June 2022 and September 2023 at two academic medical centers in Boston, MA. Clinical data, including self-reported race, was abstracted from the electronic medical record. Patients of African American, Hispanic/Lat,ino, and Asian race were categorized as minorities. Outcomes of interest were the proportion of patients with ≥50% decrease in PSA levels during LuPSMA therapy (PSA50) and overall survival, defined from the date of the first cycle of LuPSMA. PSA50 and overall survival between races were compared with the Chi-Square and log-rank tests, respectively.
A total of 159 patients were included in this study with a median age at LuPSMA initiation of 73 years (range 53-92) and a median number of prior lines of therapy of 5 (range 2-12). All patients had received ≥1 taxane and ≥1 androgen receptor pathway inhibitor. There were 139 White patients, 10 African American, 6 Hispanic/Latino, and 4 Asian patients. The median number of LuPSMA cycles received in the entire cohort was 4 (range 1-6), and median follow-up was 7.1 months: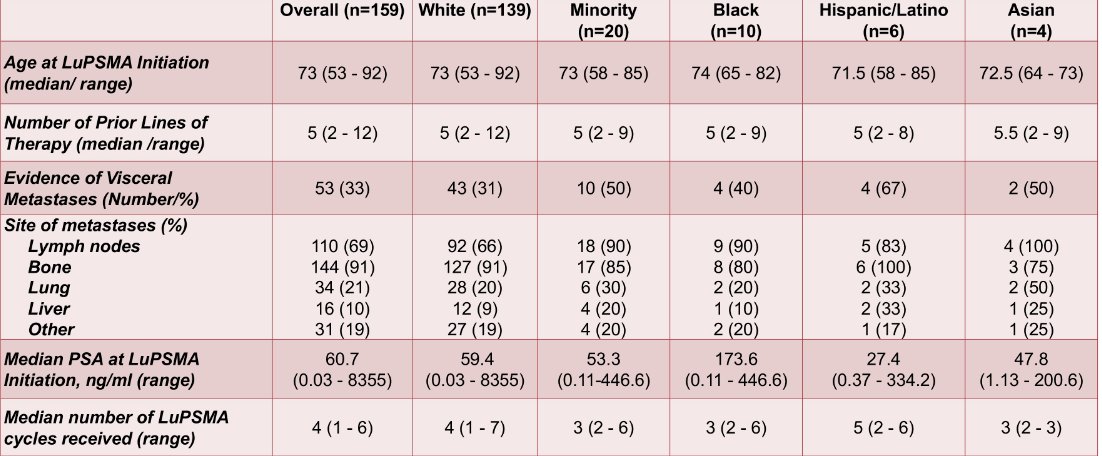
A numerically higher proportion of minority patients had visceral disease at baseline compared to White patients (50% vs. 31%, (X2 = 2.87, p = 0.090)). Overall, 86 patients (54%) had a PSA50, with PSA50 of 54% (n = 75) in White patients, 60% (n = 6) in African American, 67% (n = 4) in Hispanic/Latino patients, and 25% (n = 1) in Asian patients. There was no significant difference in PSA50 between White and minority patients (X2 = 0.00, p = 0.990). The median overall survival in the entire cohort was 12.9 months and was not significantly different between White and minority patients (12.9 months vs. 8.5 months, p = 0.533):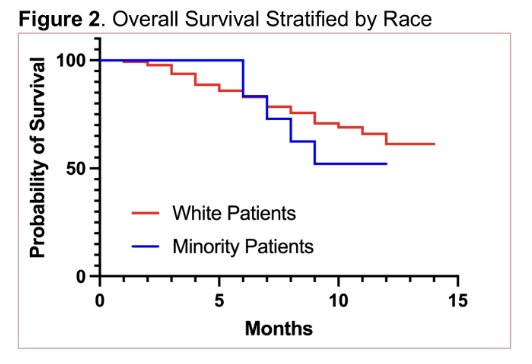
Avina Rami concluded her presentation discussing the impact of race on outcomes to LuPSMA for mCRPC with the following take-home points:
- Minority patients tend to present with more visceral disease, a known adverse prognostic factor
- PSA50 and overall survival were comparable between minority and White patients receiving LuPSMA for mCRPC, despite minority patients tending to present with more visceral disease, a known adverse prognostic factor
- Validation of these findings and efforts to widen access to LuPSMA amongst minorities are required, given that patients of all racial backgrounds appear to benefit equally from LuPSMA
Presented by: Avina Rami, Harvard Medical School, Boston, MA
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the Genitourinary (GU) American Society of Clinical Oncology (ASCO) Annual Meeting, San Francisco, CA, Thurs, Jan 25 – Sat, Jan 27, 2024.
References:


