(UroToday.com) The 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) cancers symposium held in San Francisco, CA between January 25th and 27th was host to a prostate cancer trials in progress poster session. Dr. Guillaume Grisay presented EORTC GUCG 2238 De-escalate, a pragmatic trial that aims to revisit intermittent androgen deprivation therapy (ADT) in patients with metastatic hormone-naïve prostate cancer in the era of new androgen receptor pathway inhibitors. Dr. Giulio Francolini presented PERSIAN, a randomized trial of apalutamide and stereotactic body radiation therapy (SBRT) for low-burden, metastatic, hormone-sensitive prostate cancer (mHSPC).
The TITAN trial has previously demonstrated that the addition of apalutamide to ADT improves overall survival in mHSPC patients with either low- or high-volume disease.1,2 The PERSIAN trial aims to evaluate the hypothesis of whether the addition of metastases-directed SBRT to ADT + apalutamide improves outcomes for select subgroups of patients treated with apalutamide + ADT.
This is a prospective phase II randomized trial that will include patients with metachronous oligometastatic HSPC, defined as the presence of <5 non-visceral metastatic lesions. Patients with synchronous (i.e, de novo) metastatic disease are study ineligible. In this superiority trial, patients will be randomized to:
- Apalutamide + ADT
- Apalutamide + ADT + SBRT to all sites of metastatic spread
The primary endpoint of the trial is the rate of patients with complete biochemical response, defined as PSA <0.2 ng/ml, six months following initiation of ADT + apalutamide. The target sample size is 180 patients.
At enrollment/randomization, baseline blood samples will be tested for PIK3CA/AKT1/PTEN and BRCA1/2 mutations, in order to evaluate the prognostic/predictive value of such mutations. Furthermore, oncometabolite expression will be evaluated through metabolomic analysis before and after start of SBRT
To date, enrollment is as follows: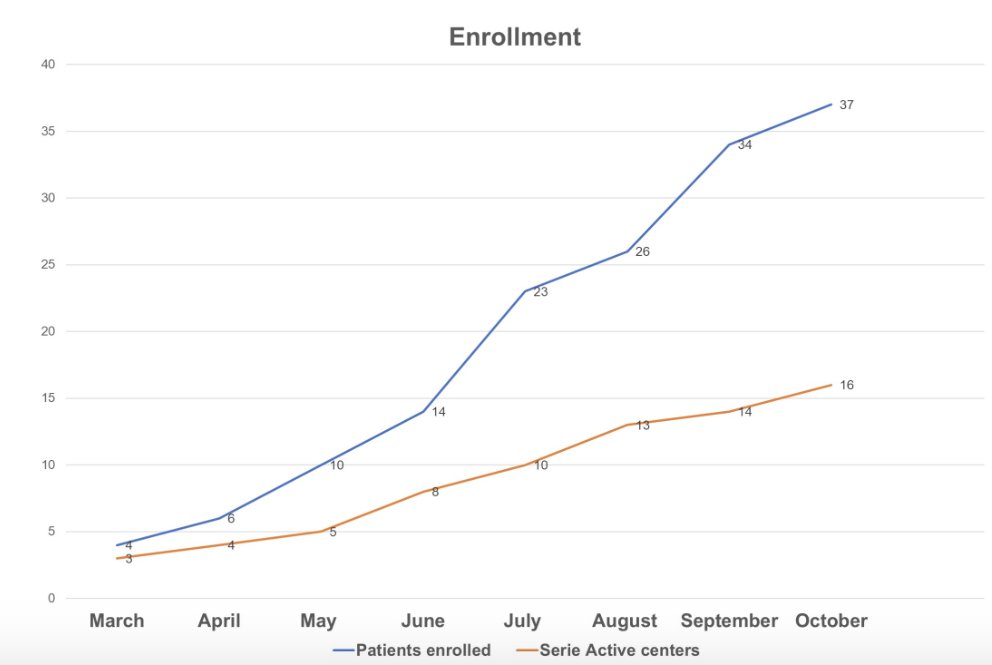
Presented by: Giulio Francolini, MD, Radiation Oncology Unit, Azienda Ospedaliera Universitaria Careggi, University of Florence, Florence, Italy
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, San Francisco, CA, January 25th – January 27th, 2024
References:- Chi KN, Agarwal N, Bjartell A, et al. Apalutamide for metastatic, castration-sensitive prostate cancer. N Engl J Med 2019 Jul 4;381(1):13-24.
- Chi KN, Chowdhury S, Bjartell A, et al. Apalutamide in patients with metastatic castration-sensitive prostate cancer: Final survival analysis of the randomized, double-blind, phase III TITAN study. J Clin Oncol. 2021 Jul 10;39(20):2294-2303.


