(UroToday.com) The 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) cancers symposium held in San Francisco, CA between January 25th and 27th was host to a prostate cancer trials in progress poster session. Dr. Evan Yu presented the study framework for a phase 1/2 study of ONCT-534, a dual-action androgen receptor inhibitor, in patients with metastatic castration-resistant prostate cancer (mCRPC).
Current therapeutic strategies for mCRPC include treatment with next-generation androgen receptor pathway inhibitors (ARPIs), which target the ligand-binding domain (LBD) of the androgen receptor (AR). Approximately 5 – 10% of patients will experience primary resistance to ARPIs and most initial responders will eventually develop resistance within 1 – 3 years. Eventually, nearly all men with prostate cancer treated with ARPIs develop resistance via several mechanisms including:
- Activation of AR genomic alterations
- Epigenetic alterations
- Expression of truncated, constitutively active AR splice variants
An ARPI switch (i.e., treatment with another ARPI after developing ARP| resistance) yields only modest clinical benefits in this setting.1 Other salvage therapies are highly toxic and impact quality of life. As such, there is a substantial unmet need for treatment of mCRPC men resistant to ≥1 ARPI.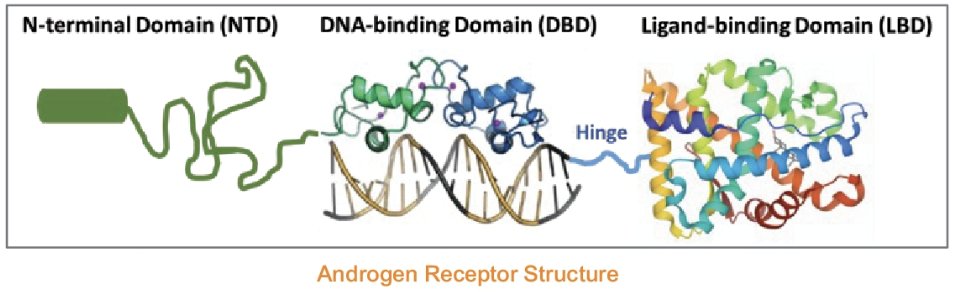
ONCT-534 is a Dual-Action Androgen Receptor Inhibitor (DAARI) with a novel mechanism of action that combines inhibition of AR function with degradation of the AR protein. This activity includes interaction with both the LBD and the N-terminal domain (NTD) of the AR, rendering it effective against splice variants and LBD mutants, unlike existing ARPIs, while also being active against native AR.
ONCT-534-101 is a phase 1/2, multi-center study that aims to evaluate the safety, tolerability, antitumor activity, and pharmacokinetics of ONCT-534 in subjects with mCRPC, without neuroendocrine differentiation or small cell features, who have relapsed or are refractory to at least one next-generation ARPI.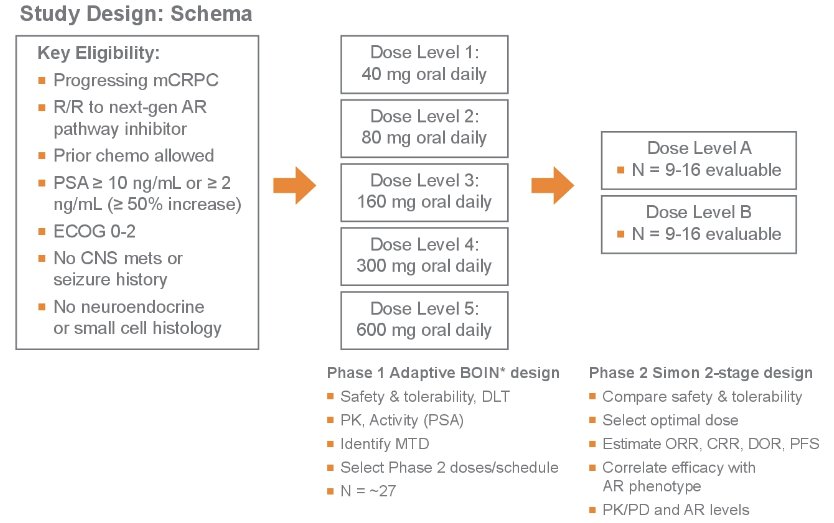
The study will be separated into a Phase 1 dose escalation and a Phase 2 dose expansion potion. The Phase 1 portion will evaluate approximately 27 subjects in 5 dose levels using an adaptive Bayesian Optimal Interval (BOIN) design to assess safety, tolerability, and DLT at escalating doses and to determine the MTD and inform the two dose levels or schedules to be tested in Phase 2. The Phase 2 portion will evaluate approximately 32 subjects in two randomized cohorts and will assess the following:
- Safety and tolerability of ONCT-534
- Compare the two different dose levels or schedules to select the optimal dose for further study
- Assess the preliminary antitumor activity of ONCT-534
In both phases, after a screening period, eligible subjects with mCRPC will receive their assigned dose regimen of ONCT-534, which will be administered orally daily for two years or until disease progression and no longer clinically benefiting from treatment or development of unacceptable toxicity.
The key eligibility criteria are summarized below:
The study endpoint are as follows: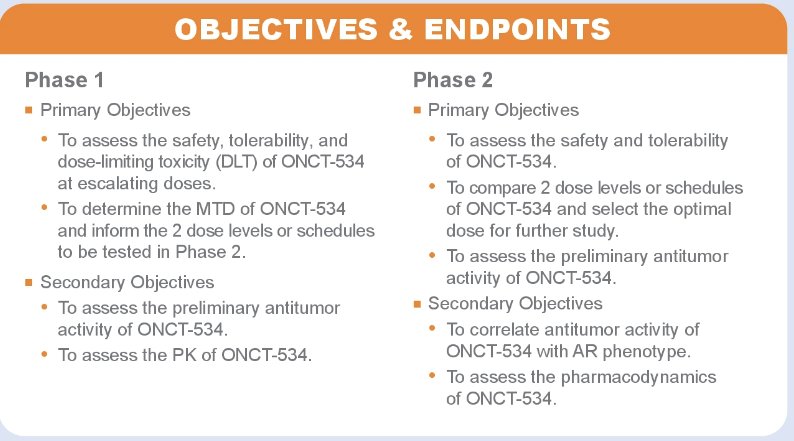
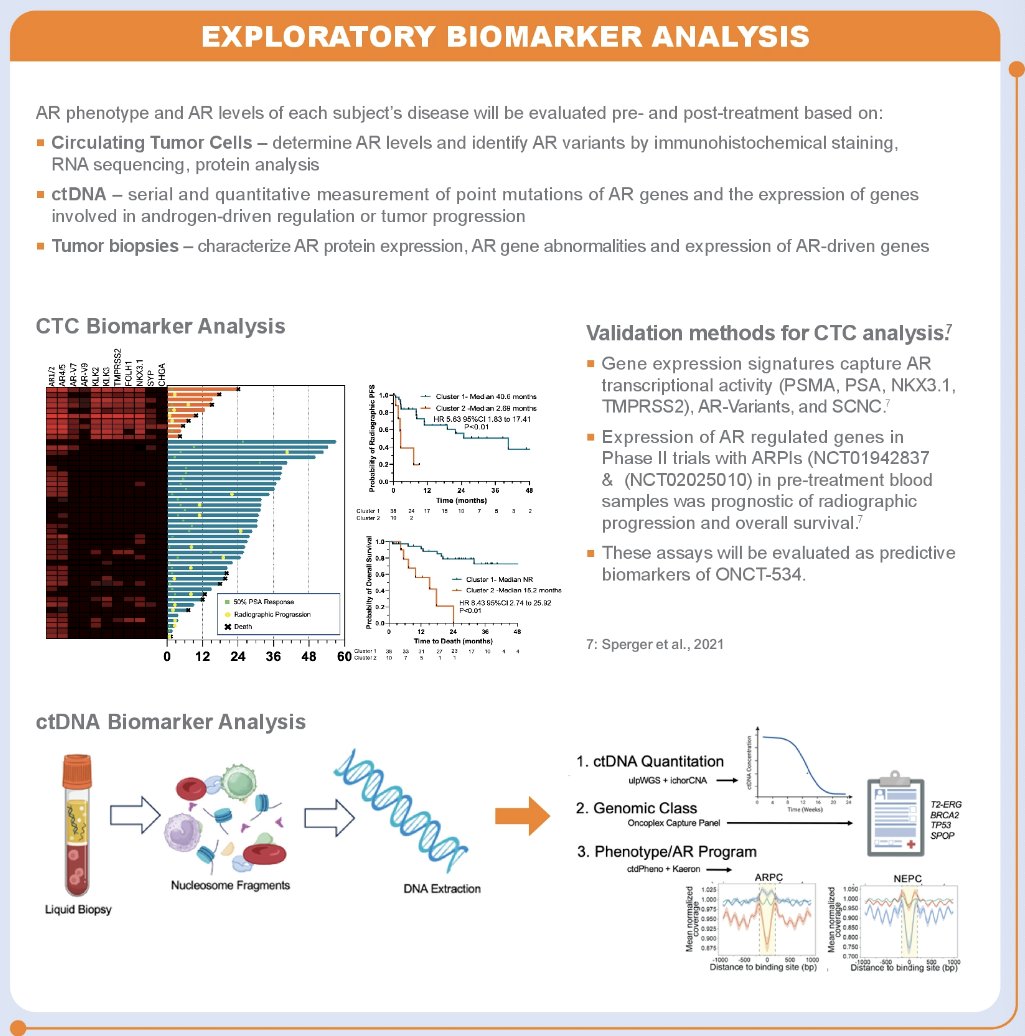
The study was opened for enrollment September 2023 and is currently active and enrolling patients in the Phase 1 dose escalation portion of the study.
Presented by: Evan Yu, MD, Professor, Department of Medicine, University of Washington and Fred Hutchinson Cancer Research Center, Seattle, WA
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, San Francisco, CA, January 25th – January 27th, 2024
References:

