(UroToday.com) The 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) cancers symposium held in San Francisco, CA was host to a prostate cancer poster session. Dr. Daniel George presented a study of real-world homologous recombination repair mutation (HRRm) testing patterns in patients with metastatic castration-resistant prostate cancer (mCRPC) treated with olaparib in the United States.
Nearly a quarter of mCRPC patients are reported to have a homologous recombination repair gene mutation (HRRm).1 Accordingly, germline and somatic testing are recommended for mCRPC patients to detect those with HRRm. This has important prognostic implications for survival and treatment selection. Olaparib is one of the first targeted therapies for patients with HRRm mCRPC. The PROfound trial demonstrated that the use of olaparib in mCRPC patients with disease progression following an androgen receptor pathway inhibitor (ARPI) improved overall survival and progression-free survival, compared to physician's choice of enzalutamide or abiraterone.2,3
The objective of the study was to describe the baseline patient characteristics, patterns of HRRm testing, and patterns of individual HRRm among mCRPC patients treated with olaparib monotherapy following prior treatment with enzalutamide and/or abiraterone and who had a positive HRRm status.
This was a retrospective, non-interventional study using electronic medical record (EMR) data from patients with a prostate cancer diagnosis date between 1990 and 2023. The investigators used the ConcertAl Oncology Dataset, which consists of EMR data available to ConcertAI through data sharing agreements with practices and other data providers. The ConcertAl Oncology Database consists of de-identified data including structured, as well as unstructured data (text and image documents, e.g., physician progress notes). The data are drawn from geographically diverse practice locations within the USA and are primarily community oncology practices (80-90%) from both rural and urban centers. Descriptive statistics were used to evaluate patient characteristics and biomarker testing patterns. Patients were grouped into two categories:
- Any BRCA mutation (including co-occurring mutations)
- Non-BRCA HRR mutations (including co-occurring mutations).
A total of 192 mCRPC olaparib-treated patients with ≥1 HRRm were identified. The overall median age was 73 years at index date. More than half (77%) of the overall sample had an ECOG performance status of 0 – 1, and 66% had a Gleason score of ≥8 at index date. Most patients (89%) in the overall sample had bone metastasis at index, and 20% had ≥3 sites of distant metastasis. The median PSA at index date was 72.2 ng/mL. Nearly one-third of patients (31%) had documented opioid use at index date. Most patients (73%) were diagnosed with CRPC in 2018 and after.
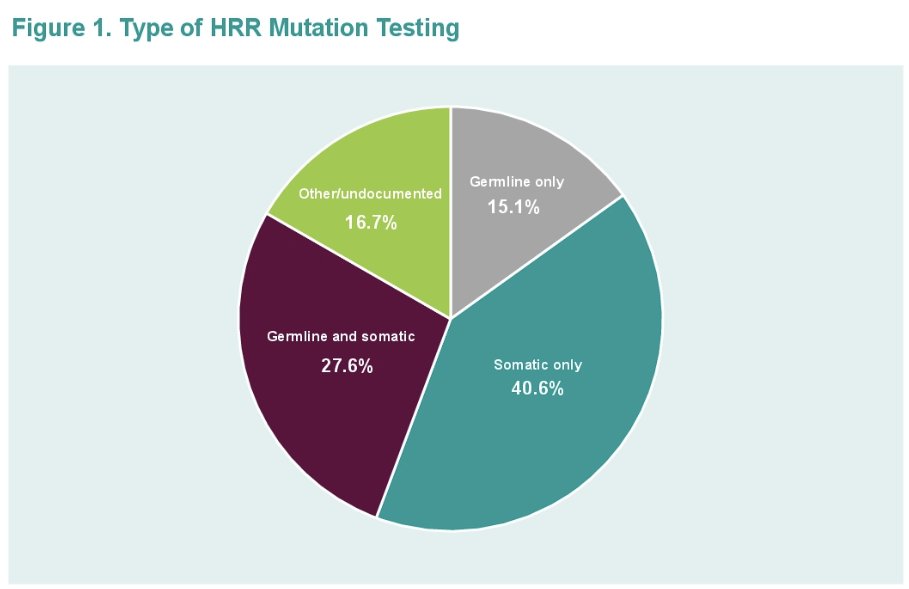
41% of patients had somatic-only testing, while 15% had germline-only testing. 28% of patients had both germline and somatic testing, and 17% had other/undocumented/undetermined testing. (Figure 1)
Among patients with any BRCA mutation:
- 36.1% had somatic only testing
- 18.5% had germline only testing
- 28.7% had both germline and somatic testing
- 16.7% had other/undocumented/undetermined testing
Among patients with non-BRCA HRR mutation:
- 46.4% had somatic only testing
- 10.7% had germline only testing
- 26.2% had both germline and somatic testing
- 16.7% had other/undocumented/undetermined testing
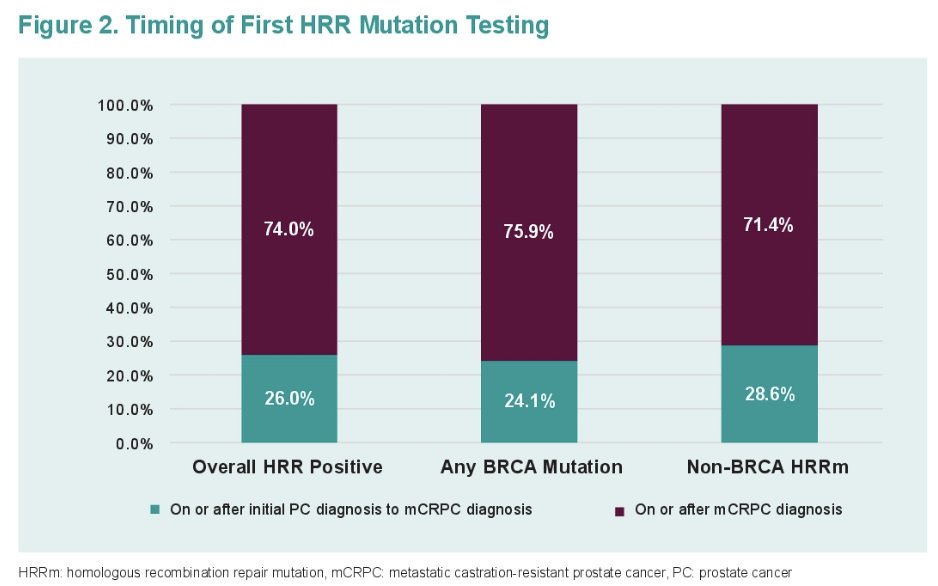
Most patients were tested on or after mCRPC diagnosis (74%). Only 6% of patients tested on or after mCRPC diagnosis were tested prior to 1st line treatment.
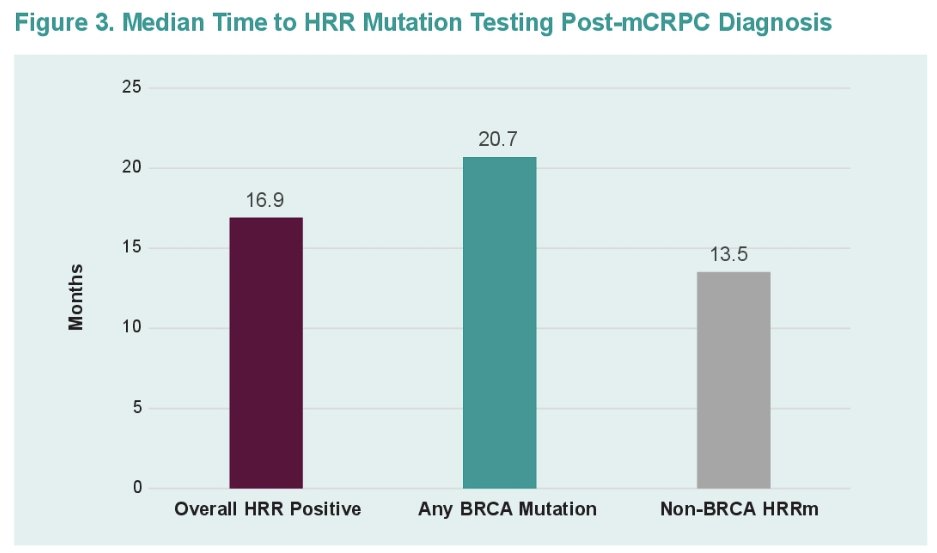
Among BRCA mutation patients, the median time from mCRPC diagnosis to biomarker testing was 20.7 months. Only 3.7% of patients tested after mCRPC diagnosis were tested prior to 1st line treatment. The corresponding values for non-BRCA HRR were 13.5 months and 13.3%, respectively.
The median time from mCRPC diagnosis to the first biomarker test was 16.9 months.
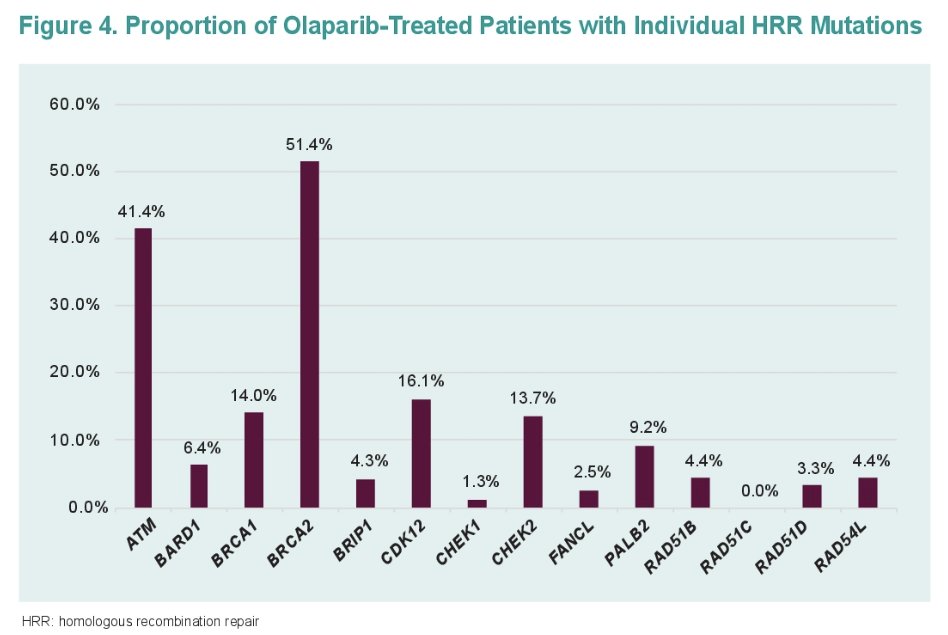
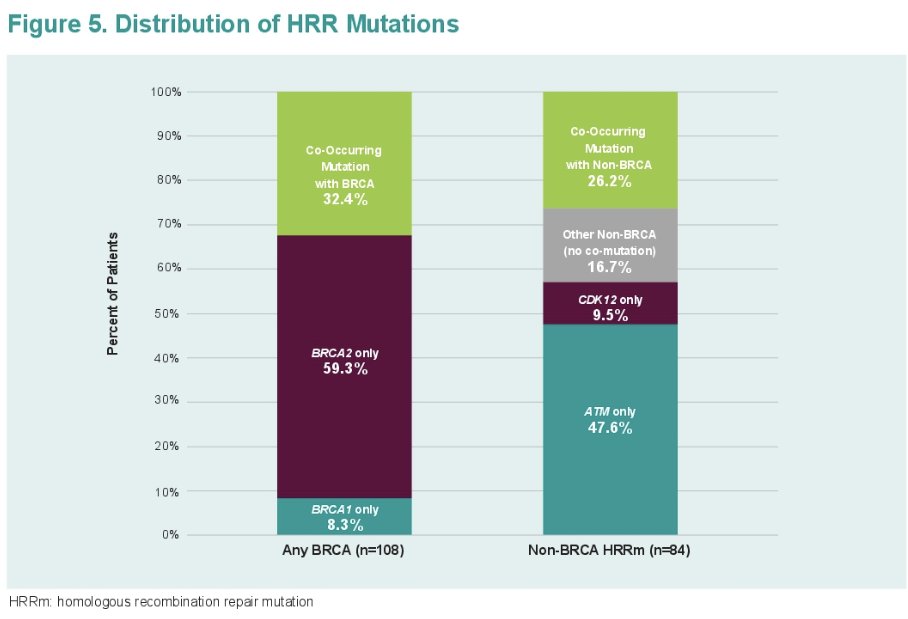
Over half of the patients (56%) had any BRCA mutation (BRCA2: 33.3%, BRCA1: 4.7%, co-occurring HRRm: 18.2%). 43.8% had non-BRCA HRR mutation (ATM: 20.8%. CDK12: 4.2%, co-occurring HRRm 11.5%, other non-BRCA HRR: 7.3%).
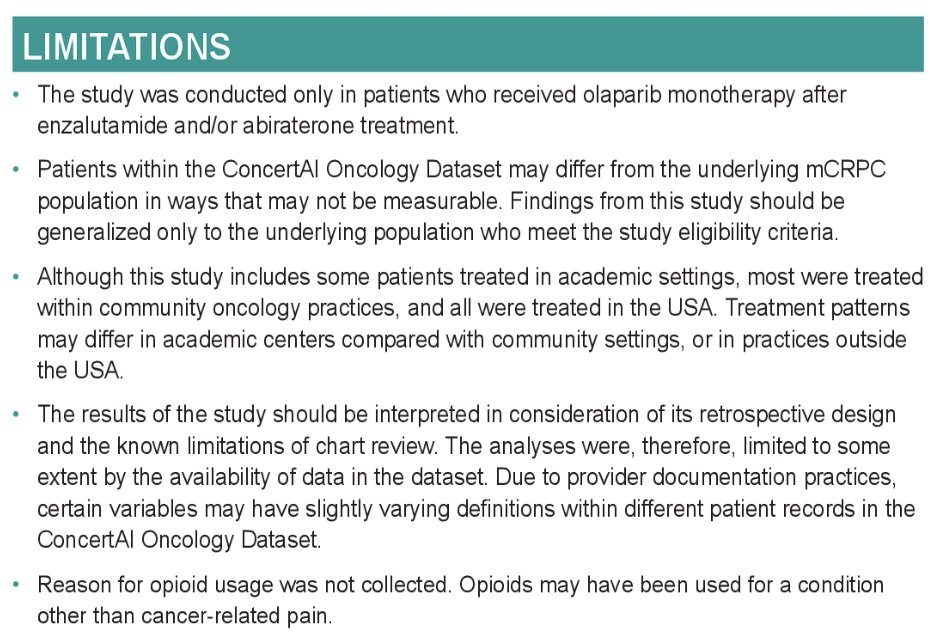
Limitations to this study include the following:
Dr. George concluded that:
- This real-world analysis highlights the need for earlier and more consistent HRRm testing in patients with mCRPC to allow for optimal timing of novel treatment options that have shown efficacy in a biomarker-selected population.
- Most patients in this study were tested and diagnosed with HRRm many months after mCRPC diagnosis, at which point they had high levels of bone metastasis, multiple sites of distant metastases, and opioid use at the initiation of olaparib treatment with less likelihood of benefiting from a precision oncology approach.
- HRR testing in patients before or at the time of mCRPC would allow for olaparib therapy earlier in the disease course and potentially greater clinical benefit. Based on an expanded FDA label, earlier testing will be needed in order to utilize olaparib with abiraterone in patients with BRCA mutations in the 1st line mCRPC setting.
Presented by: Daniel J. George, MD, Professor of Medicine, Department of Medicine, Co-Chair, DCI Center for Prostate & Urologic Cancers; Director of Genitourinary Oncology at Duke Cancer Institute, Durham, NC
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, San Francisco, CA, January 25th – January 27th, 2024
References:
- Pritchard CC, Mateo J, Walsh MF, et al. Inherited DNA-Repair gene mutations in men with metastatic prostate cancer. N Engl J Med. 2016;375(5):443-453.
- de Bono J, Mateo J, Fizazi K, et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med 2020 May 28;382(22):2091-2102.
- Hussain M, Mateo J, Fizazi K, et al. Survival with Olaparib in Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2020 Dec 10;383(24):2345-2357.