(UroToday.com) The 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) cancers symposium held in San Francisco, CA was host to a prostate cancer poster session. Dr. Neal Shore presented the results of a systematic review evaluating whether the findings of indirect treatment comparisons in metastatic hormone sensitive prostate cancer (mHSPC) are consistent.
Darolutamide in combination with docetaxel plus androgen-deprivation therapy (ADT) is US FDA approved for the treatment of mHSPC patients. The phase 3 ARASENS trial demonstrated that the addition of darolutamide to docetaxel + ADT produced clinically significant overall survival improvements.1,2
Many indirect treatment comparisons (ITCs) have been published since that have compared the efficacy and safety of treatment alternatives in the mHSPC disease space – agents that have not been directly compared in head-to-head clinical trials. Given the variety of publications in this field, with the inherent methodological heterogeneity among ITCs, it is difficult to distinguish whether findings are consistent and what limitations there are to this evidence. As such, the objective of this study was to assess the findings of previous published ITCs, focusing on outcomes related to triplet therapy with darolutamide + docetaxel + ADT from ARASENS.1,2
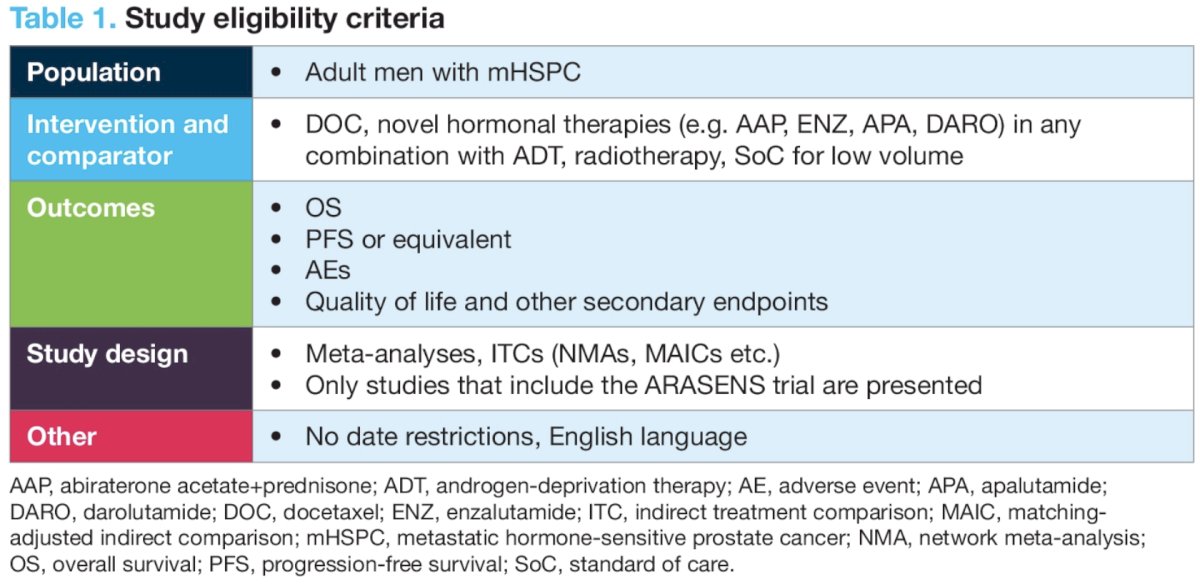
Dr. Shore and colleagues performed a systematic literature review of MEDLINE, Embase, and Cochrane to identify ITCs published between 1946 and May 2023 (PROSPERO Registration: CRD42023429478). They focused on reporting ITCs that included ARASENS trial data and treatment versus treatment comparisons. The study eligibility criteria are summarized below:
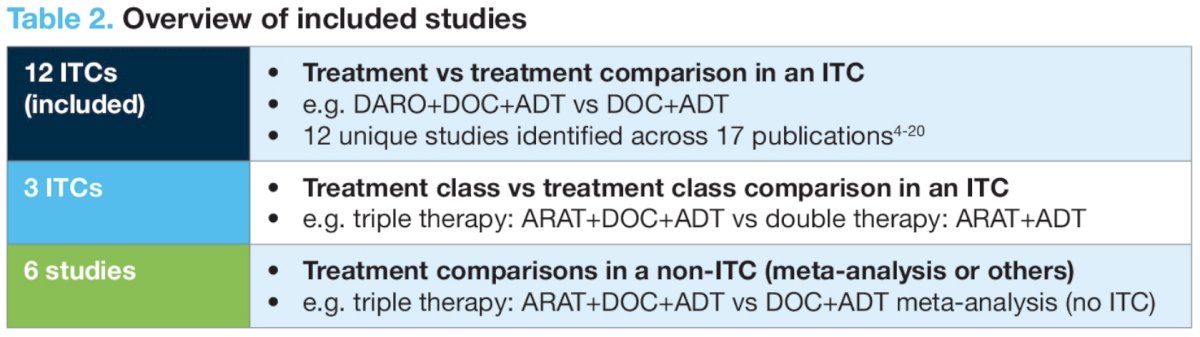
The analysis included 12 ITCs from 17 publications.

There were variations across the ITCs with respect to:
- The number of trials included (range: 2 to 10)
- Statistical methods used (Frequentist or Bayesian network meta-analyses, random or fixed effects modeling)
- Reporting and testing of treatment effect modifiers, network inconsistency/heterogeneity and subgroup analysis methodology.
The ITCs consistently demonstrated a significant benefit in OS with darolutamide triplet therapy, compared with ADT alone (4 ITCs) and with docetaxel + ADT (7 ITCs). Darolutamide triplet therapy demonstrated a numerical benefit in OS compared with:
- Abiraterone acetate + prednisone (AAP)+ADT, enzalutamide (ENZ) + ADT and apalutamide (APA) + ADT
- AAP triplet therapy
Darolutamide triplet therapy was consistently ranked highest, above other androgen receptor axis-targeted (ARAT) doublet and triplet therapies, on OS across 9 ITCs.
The risk of grade ≥ 3 adverse events (AEs) was numerically greater with darolutamide triplet therapy compared with APRI doublets (6 ITCs; low to moderate certainty). There were numerically similar grade ≥ 3 AEs with darolutamide triplet therapy compared with docetaxel doublet therapy across 4 ITCs (low to moderate certainty). Available ITC evidence comparing AEs across triplet therapies showed numerically fewer grade ≥ 3 AEs for darolutamide compared with AAP (2 ITCs; low certainty).
Dr. Shore concluded that:
- Darolutamide triplet therapy ranked highest in terms of providing an OS benefit.
- Darolutamide triplet therapy was associated with a lower rate of grade ≥ 3 AEs compared with AAP triplet therapy.
- Despite variability, ITCs showed consistent findings for OS. However, there is a need to standardize ITC methodology for safety endpoints to ensure robustness and interpretability of findings and to optimize treatment decision-making.
Presented by: Neal Shore, MD, FACS, Urologist, Director, CPI, Carolina Urologic Research Center, Atlantic Urology Clinics, Myrtle Beach, SC
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, San Francisco, CA, January 25th – January 27th, 2024
References:
- Smith MR, Hussain M, Saad F, et al. Darolutamide and Survival in Metastatic, Hormone-Sensitive Prostate Cancer. N Engl J Med. 2022 Mar 24;386(12):1132-1142.
- Hussain M, Tombal B, Saad F, et al. Darolutamide plus androgen-deprivation therapy and docetaxel in metastatic hormone-sensitive prostate cancer by disease volume and risk subgroups in the phase III ARASENS trial. J Clin Oncol. 2023 Jul 10;41(20):3595-3607.