(UroToday.com) The 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) cancers symposium held in San Francisco, CA was host to a prostate cancer poster session. Dr. Junlong Zhuang presented the results of a multi-center, open-label, single-arm phase II trial evaluating neoadjuvant darolutamide plus androgen deprivation therapy (ADT) for patients with high- or very high-risk, localized prostate cancer.
Patients with high- and very high-risk, localized prostate cancer undergoing single modality treatment with radical prostatectomy remain at high-risk of biochemical failure. There has long been interest in evaluating neoadjuvant treatment options to improve survival outcomes in such patients. This study aimed to evaluate the efficacy and safety of darolutamide, an androgen receptor pathway inhibitor (ARPI), plus androgen deprivation therapy (ADT) for patients with locally advanced prostate cancer undergoing radical prostatectomy.
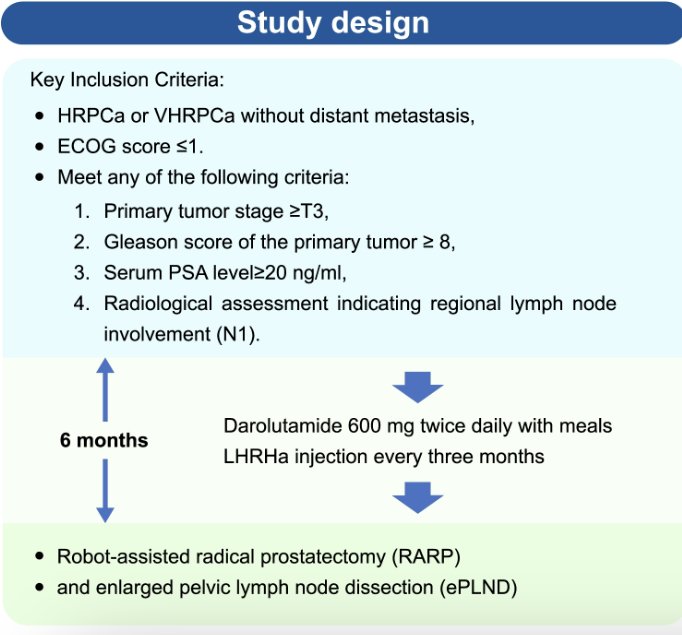
This was a multicenter, single-arm, open-label phase II clinical trial (NCT05249712). Eligible patients received six months of neoadjuvant darolutamide + ADT, followed by surgery. The efficacy outcomes included:
- Pathologic complete response (pCR)
- Minimal residual disease (MRD)
- Positive surgical margin status
- Progression-free survival
- Stage downgrading
Circulating tumor DNA (ctDNA) analysis and next-generation sequencing (NGS) were used to evaluate the correlation between systemic disease burden and gene mutations with neoadjuvant response, respectively. Safety events were also collected and reported.
The study cohort consisted of 30 patients, with baseline characteristics summarized below.
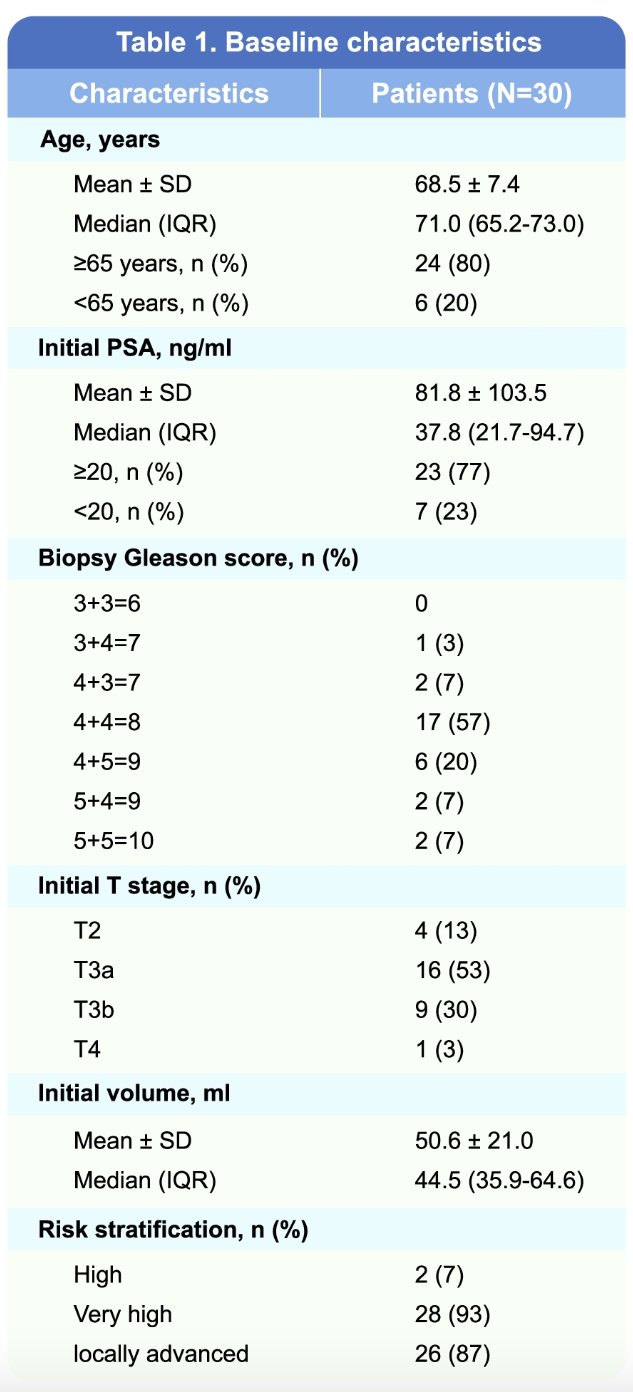
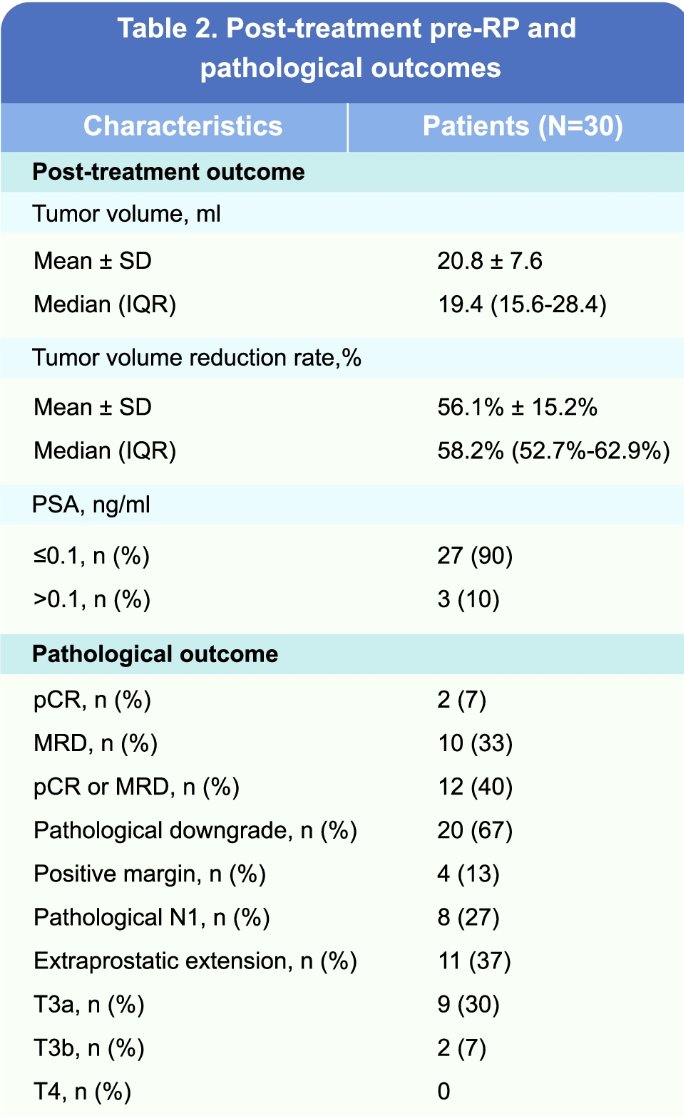
All patients received darolutamide plus ADT for six months, followed by radical prostatectomy. Pathologic outcomes were as follows:
- pCR: 6.7%
- MRD: 33.3%
- Positive surgical margins: 13.3%
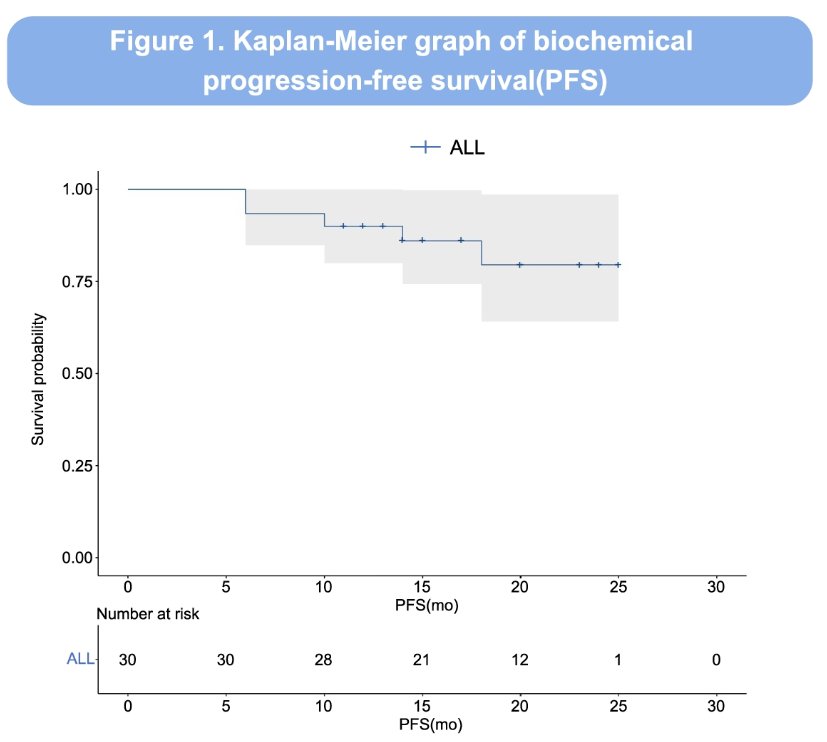
The 12 months PFS was 90% (95% CI: 74.4 – 96.5%).
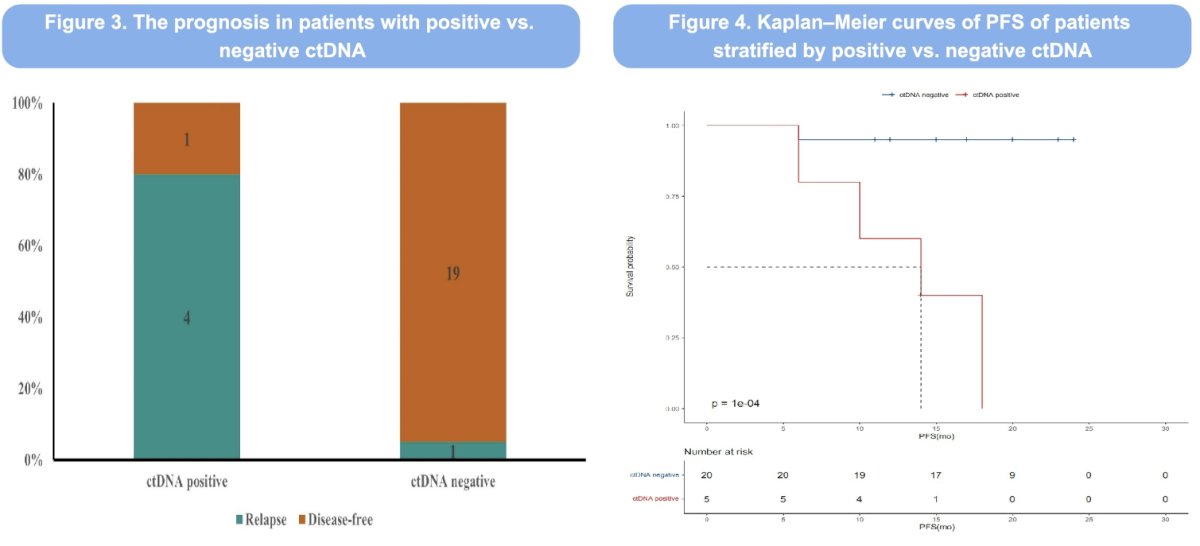
Of the 25 patients who underwent NGS testing, 22 (88%) had ≥1 mutation. The most frequently mutated gene was FOXA1 (36%). ctDNA was positive in five patients, of whom four (80%) experienced disease progression. In contrast, among the 20 patients with negative ctDNA status, only one (5%) experienced disease progression.
No grade 3 or 4 adverse events were observed throughout the trial. The most frequent adverse events included hot flashes and elevated alanine aminotransferase or aspartate transaminase levels, which were observed in three patients (10%).
Dr. Zhuang concluded that neoadjuvant therapy with darolutamide plus ADT for 6 months followed by radical prostatectomy is safe and effective for high- and very high-risk prostate cancer. ctDNA is a prognostic biomarker for disease progression in these patients.
Presented by: Junlong Zhuang, Nanjing Drum Tower Hospital, The Affiliated Hospital of Nanjing University Medical School, People’s Republic of China
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, San Francisco, CA, January 25th – January 27th, 2024