(UroToday.com) The 2024 GU ASCO annual meeting featured a prostate cancer session and a presentation by Dr. Andrei Gafita discussing response evaluation criteria in PSMA PET/CT in metastatic castration-resistant prostate cancer (mCRPC). Response Evaluation Criteria in PSMA PET/CT (RECIP 1.0) initially integrated software-based quantitative assessment of PSMA-positive total tumor volume.
However, clinical implementation of such software is not expected soon, limiting the use of RECIP in practice. The aim of this study presented at GU ASCO 2024 was to assess the agreement of RECIP determined using tumor segmentation software (quantitative RECIP) with RECIP determined by qualitative reads by nuclear medicine physicians (visual RECIP) for response evaluation in mCRPC.
This was a multicenter retrospective study at three academic centers, which included patients who received 177LuPSMA treatment between December 2014 and July 2019. PSMA PET/CT images at baseline and 12 weeks were assessed qualitatively by five nuclear medicine physicians for changes in PSMA-positive total tumor volume and for new lesions. Quantitative changes in PSMA-positive total tumor volume were also measured using tumor segmentation software. The status of new lesions was combined with qualitative changes in PSMA-positive total tumor volume to determine visual RECIP and with quantitative changes in PSMA-positive total tumor volume to determine quantitative RECIP. The study design is as follows: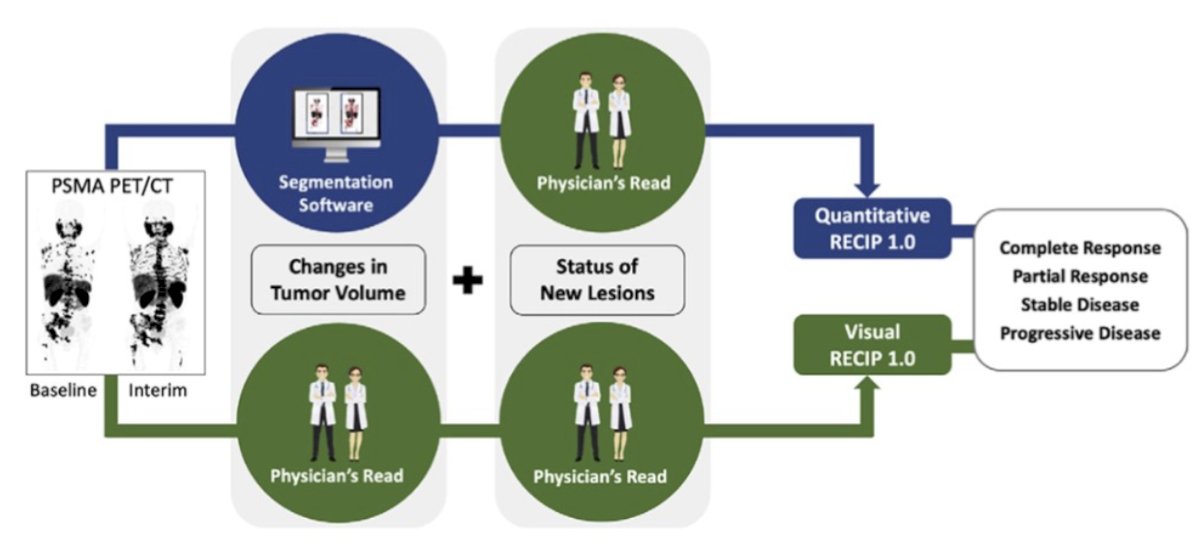
The primary outcomes were the agreement between visual and quantitative RECIP and the inter-reader reliability of visual RECIP according to the Fleiss κ. The secondary outcome was the association of visual RECIP with overall survival according to Cox regression. The definition criteria for RECIP is as follows:
Of the 287 patients screened retrospectively, there were a total of 124 patients, with a median age of 73 years (IQR, 67–76 years) that were included. There were 89/124 men (72% that received a 68Ga-PSMA-11 scan and 35/124 (28%) men that received a 18F-rhPSMA-7.3 scan. Forty (32%) and 84 (68%) patients had quantitative RECIP progressive disease and non-progressive disease, respectively. For RECIP progressive disease versus RECIP non-progressive disease, agreement among all five readers was observed in 103/124 (83%) of patients (excellent agreement, κ = 0.81) for visual RECIP and 114/124 (92%) for patients with quantitative RECIP (excellent agreement, κ = 0.92). Pairwise agreement for progressive disease and non-progressive disease is as follows: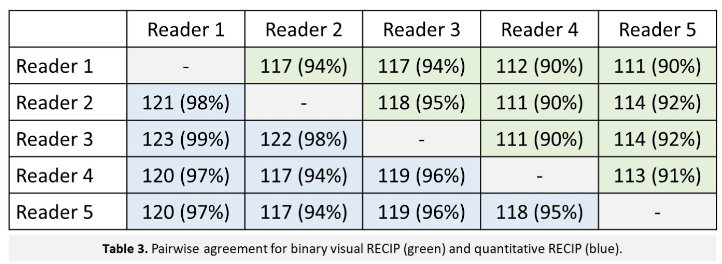
Agreement between quantitative RECIP and visual RECIP was excellent (κ > 0.81) for all readers. Individual results for progressive disease versus non-progressive disease are as follows: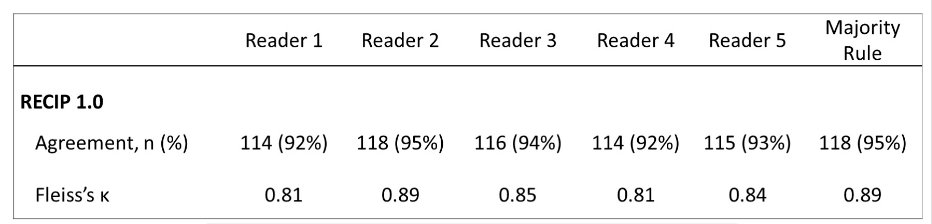
Furthermore, RECIP-progressive disease was associated with significantly shorter overall survival compared with RECIP-non-progressive disease (HR 2.6, 95% CI 1.7, 3.8).
Dr. Gafita concluded his presentation discussing response evaluation criteria in PSMA PET/CT (RECIP 1.0) in mCRPC with the following take-home points:
- RECIP 1.0 assessed qualitatively by nuclear medicine physicians (visual RECIP) demonstrated excellent inter-reader reliability and excellent agreement with RECIP determined using tumor segmentation software (quantitative RECIP)
- The association of visual RECIP with overall survival is promising for use in daily practice and clinical trials
- Validation of RECIP for monitoring other systemic treatments for mCRPC is warranted
Presented by: Andrei Gafita, MD, Johns Hopkins University, Baltimore, MD
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the Genitourinary (GU) American Society of Clinical Oncology (ASCO) Annual Meeting, San Francisco, CA, Thurs, Jan 25 – Sat, Jan 27, 2024.


