(UroToday.com)The 2024 GU ASCO annual meeting included a prostate cancer session featuring trials in progress and a presentation by Dr. Nate Lenzo discussing the trial design of ProstAct-SELECT, including safety, tolerability, and dosimetry of 177Lu-TLX591 with best standard of care in patients with PSMA-expressing metastatic castration-resistant prostate cancer (mCRPC).
PSMA has proven to be an ideal therapeutic target in prostate cancer as it is highly expressed by malignant prostate cells. Based on previous clinical evidence of its favorable safety profile and specificity for prostate cancer tumors, 177Lu-DOTA-HuJ591-CHO (177Lu-TLX591) may be a potential radioimmunotherapy for the treatment of prostate cancer. There has been evidence of anti tumor effect and a clear dose-response profile for key measures of activity, including PSA response and overall survival.
ProstACT-SELECT is a phase 1 study designed to evaluate the safety, tolerability, biodistribution, and dosimetry of 177Lu-TLX591 administered with standard of care for patients with PSMA-expressing, mCRPC progressing despite prior treatment with a novel androgen axis drug. This study previously reported positive interim results, and primary analysis of safety and dosimetry data is currently underway.
Approximately 28 participants are to be enrolled in two cohorts. In cohort 1, five patients received a single (27 mCi) intravenous infusion of 177Lu-TLX591. SPECT images and pharmacokinetic blood samples will be acquired at several time points until Day 13 after dosing. Dosimetry analysis and qualitative comparison of biodistribution of tracer level of 177Lu-TLX591, as demonstrated by SPECT, with 68Ga-PSMA-11 on PET imaging were performed to ensure equivalent (or improved) radiopharmaceutical tumour targeting. Patients were to receive a second administration of 177Lu-TLX591, at a full therapeutic dose of 76 mCi, 14 days following the initial dose after safety confirmed by independent review board. Standard of care was to continue according to standard practice. In cohort 2, 23 subsequent patients were to receive 2 administrations of 76 mCi 177Lu-TLX591, with SPECT dosimetry and pharmacokinetics obtained as in cohort 1: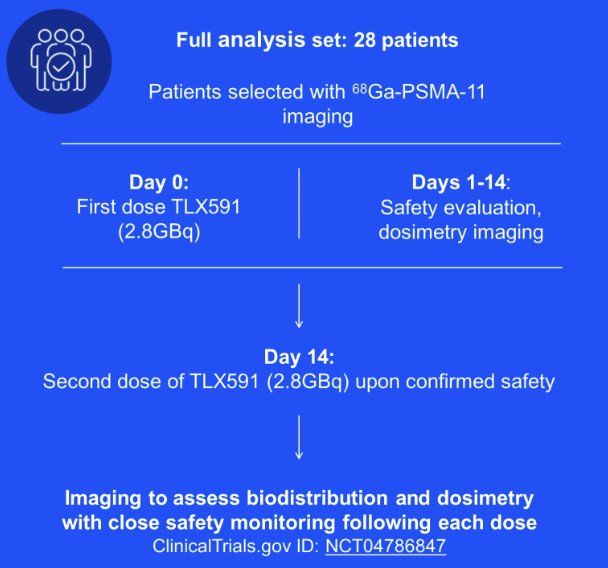
The primary endpoints include:
- Absorbed radiation dose of administered 177Lu-TLX591 to kidneys, liver, lungs, spleen, bone/red marrow, and salivary glands
- Tumour-to-healthy tissue ratios and residence times
- Type, frequency, and severity of treatment emergent adverse events
Investigation of TLX591 is continuing in the ProstACT GLOBAL phase 3 study, which is a multinational, multi-center, prospective, randomized control, open label study designed to investigate and confirm the benefits and risks associated with 177Lu-TLX591 administered together with standard of care as compared to standard of care alone in participants with PSMA-positive mCRPC.
Clinical trial information: NCT04786847
Presented by: Nate Lenzo, MD, GenesisCare, Fort Myers, FL
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the Genitourinary (GU) American Society of Clinical Oncology (ASCO) Annual Meeting, San Francisco, CA, Thurs, Jan 25 – Sat, Jan 27, 2024.


