(UroToday.com) The 2024 GU ASCO annual meeting included a prostate cancer session featuring trials in progress and a presentation by Neel Patel discussing the trial design of ProstACT GLOBAL, a phase 3 study of best standard of care with and without 177Lu-DOTA-rosopatamab (TLX591) for patients with PSMA expressing metastatic castration-resistant prostate cancer (mCRPC) progressing despite prior treatment with a novel androgen axis drug. The treatment of advanced prostate cancer is challenging, with undesirable side effects that impact patient quality of life. Radioimmunotherapy can localize therapy to specific tumor cells in multiple organs to reduce or eliminate damage to normal tissue. PSMA is an ideal therapeutic target, as it is highly expressed by malignant prostate cells, thus there is a strong rationale for further investigation of the 177Lu-labeled, chelator-conjugated antibody, 177Lu-DOTA-rosopatamab, as a potential radioimmunotherapy candidate for the treatment of prostate cancer. In phase I, ProstACT SELECT data showed excellent uptake and retention in tumor and metastases up to 14 days post-injection:
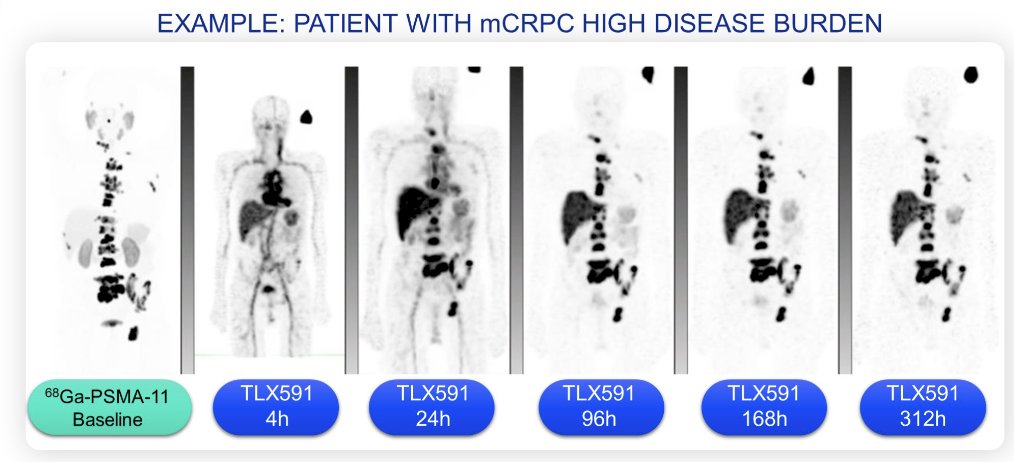
Additionally, TLX591 has had promising activity in 242 patients in phase I and II trials with up to a 42.3 month median survival.
In this multinational, multicenter, prospective, randomized, open label phase 3 study, 387 patients with PSMA-expressing mCRPC that have progressed despite prior treatment with a novel androgen axis drug will be enrolled in a 2:1 ratio to receive either the best standard of care or two single IV injections of 76 mCi each (equivalent to a 45 mCi/m2 dose in a standard 1.7m2 individual) of 177Lu-DOTA- rosopatamab, given 14 days apart, plus best standard of care. Eligible patients must have received prior therapy with either enzalutamide or abiraterone plus prednisone, and one line of prior taxane therapy or have refused or are ineligible for taxanes:
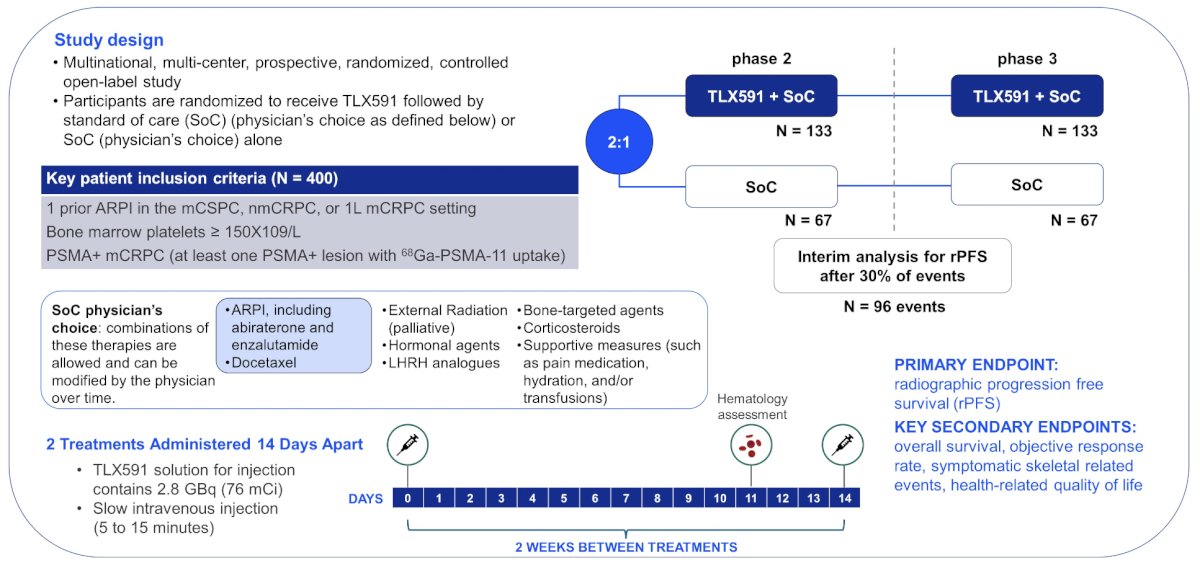
Patients must have adequate organ function including at least 150 x 109/L platelets, hemoglobin 10 g/dL, and have PSMA-positive disease on 68Ga-PSMA-11 PET/CT imaging as confirmed by a central reader. Key exclusion criteria include small cell histology, increased risk of hemorrhage or bleeding, known brain or hepatic metastases, or history of stroke, seizure, or treatment with radioisotopes within 6 months prior to randomization. The primary endpoint is radiographic progression-free survival. Secondary endpoints include:
- 5-year overall survival
- Tumor objective response rate
- Time to symptomatic skeletal event
- Progression free survival
- Number of participants with treatment-related adverse events
The key ProstACT GLOBAL phase /3 protocol assessments and elements are as follows:

Clinical trial information: NCT04876651.
Presented by: Neel Patel, DPhil, BM, BCh, Telix Pharmaceuticals, Indianapolis, IN
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, San Francisco, CA, January 25th – January 27th, 2024


