(UroToday.com) The 2024 GU ASCO annual meeting featured a prostate cancer session and a presentation by Dr. Benjamin Lowentritt discussing real-world comparison of PSA response in patients with metastatic castration-sensitive prostate cancer (mCSPC) treated with apalutamide or enzalutamide. Deep PSA response, evaluated as ≥90% PSA decline (PSA90), is an important outcome after androgen receptor synthesis inhibitor initiation, with early, deep response associated with longer survival.
Apalutamide1 and enzalutamide,2-3 two androgen receptor synthesis inhibitors, used in combination with ADT are among the recommended treatments for patients with mCSPC. A prior real-world study in community based urology practices in the United States found that apalutamide was associated with 56% higher PSA90 response rates than enzalutamide among patients with mCSPC at 6-months post-treatment initiation, as verified by medication receipt with in-office dispensing.4 The primary objective of this study presented at the GU ASCO 2024 annual meeting was to compare PSA90 response in androgen receptor synthesis inhibitor-naïve patients with mCSPC by 6 months after initiating apalutamide or enzalutamide in real-world clinical practice.
Linked electronic medical record data of US community urology practices in the PPS Analytics and administrative health data from Komodo Health Solutions Research Database were evaluated. Patients with mCSPC were selected into two cohorts, based on the index date, defined as the first paid pharmacy claim or in-office dispensation for apalutamide or enzalutamide on or after December 16, 2019. Patients were required to have ≥12 months of pre-index electronic medical record clinical activity. Pre-index characteristics (for example, age, race, geographic region, payer type, index year, time from metastasis, time from first prostate cancer diagnosis, de novo prostate cancer, ADT use, first-generation antiandrogen use, chemotherapy use, metastasis locations, most recent PSA level, testosterone level, and Gleason score) were balanced between cohorts with inverse-probability treatment weighting (IPTW). Patients were followed from index date until the earliest of index androgen receptor synthesis inhibitor discontinuation or switch, radiopharmaceutical initiation, end of open administrative claim or electronic medical record clinical activity, or end of data availability (September 30, 2022). PSA90 was defined as the earliest ≥90% decline in PSA relative to most recent pre-index PSA. The proportion of patients achieving PSA90 was compared using a weighted Kaplan-Meier analysis and the time-to-PSA90 response was compared using a weighted Cox proportional hazards model.
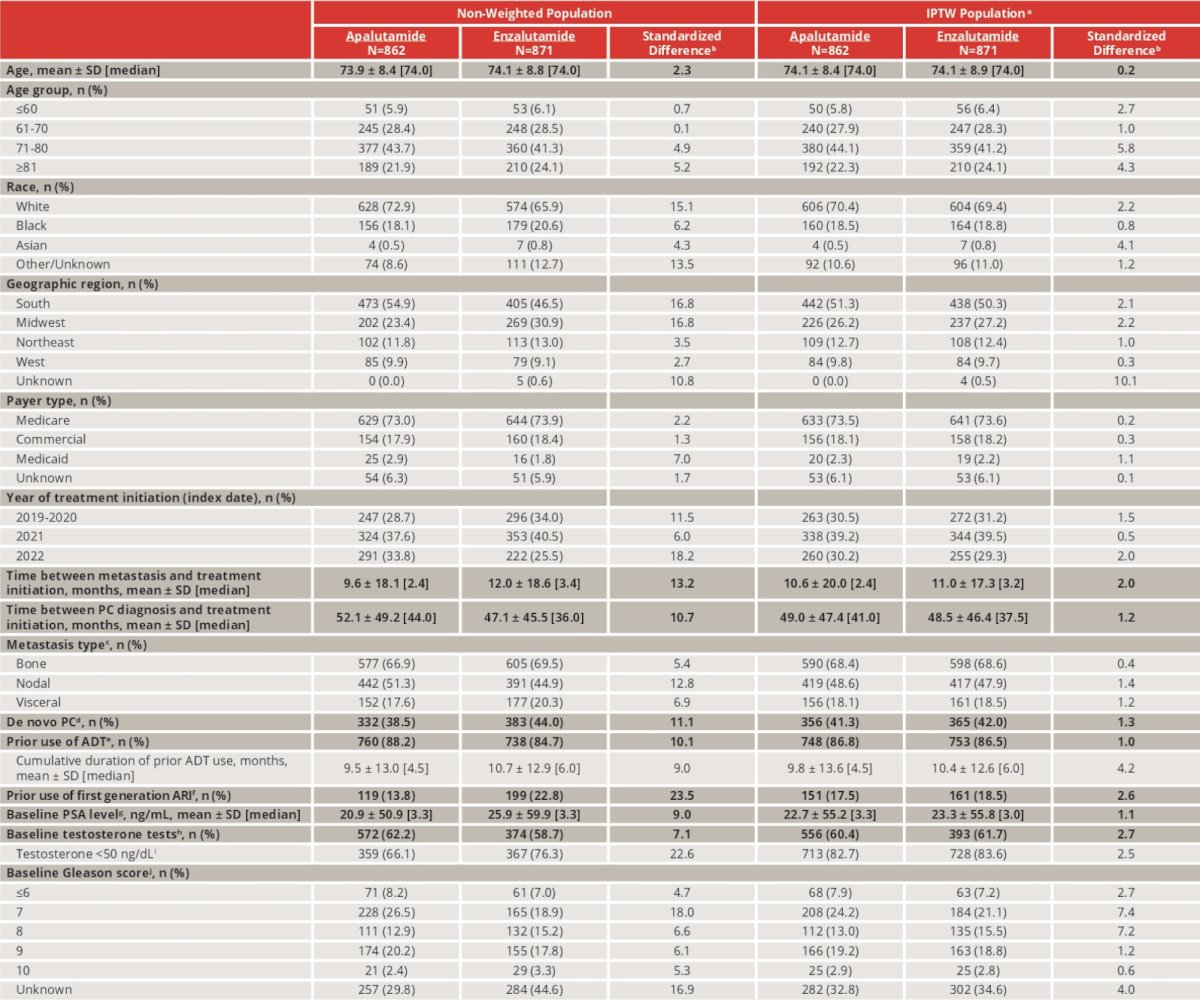
In total, there were 862 apalutamide and 871 enzalutamide patients identified for this study. Patient pre-index characteristics were balanced with IPTW:
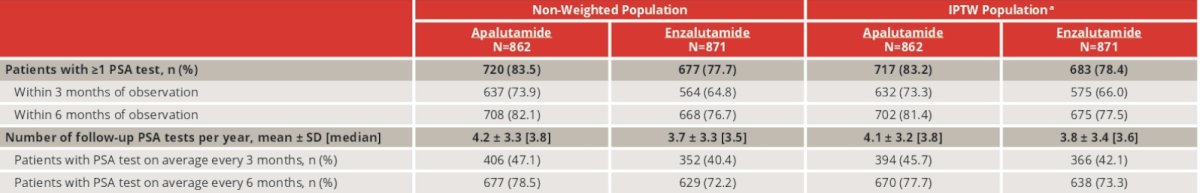
PSA testing occurred more frequently among patients in the apalutamide cohort than in the enzalutamide cohort. By 6 months post-index, 81.4% of apalutamide and 77.5% of enzalutamide patients had ≥1 PSA test result:
By 6 months post-index, apalutamide patients were 21% more likely to achieve a PSA90 response compared with similar patients initiated on enzalutamide (HR 1.21, 95% CI 1.05-1.40):
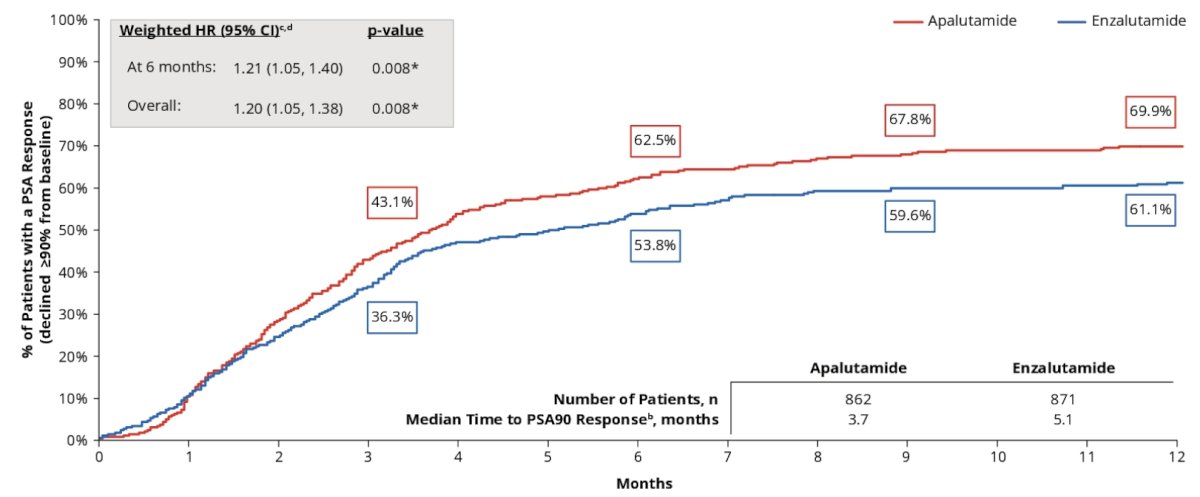
The same trend was observed over the entire observation period (p = 0.008). PSA90 response was attained earlier in patients treated with apalutamide than for those treated with enzalutamide, with the median time to PSA90 of 3.7 months for apalutamide versus 5.1 months for enzalutamide.
Dr. Lowentritt concluded his presentation discussing real-world comparison of PSA response in patients with mCSPC treated with apalutamide or enzalutamide with the following take-home points:
- In patients with mCSPC, a higher proportion of patients treated with apalutamide attained a PSA90 response than those treated with enzalutamide
- PSA90 response was attained earlier in patients treated with apalutamide than those treated with enzalutamide
- These findings confirm results from a prior study that reported higher rates of PSA90 for apalutamide initiators than enzalutamide initiators who were treated through community-based urology practices
- The proportions of patients with PSA90 response by 12 months following initiation of apalutamide in this real world study are consistent with those observed in patients with mCSPC enrolled in the TITAN study
- The clinical implications of these observations warrant further consideration given existing evidence on the association between attainment of rapid and deep PSA response with survival-related endpoints in patients treated with these medications
Presented by: Benjamin H. Lowentritt, MD, FACS, Chesapeake Urology, Baltimore, MD
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, San Francisco, CA, January 25th – January 27th, 2024
Related content: Analyzing mHSPC Patient Responses to ADT with Enzalutamide vs. Apalutamide - Benjamin Lowentritt
References:
- Chi KN, Agarwal N, Bjartell A, et al. Apalutamide for metastatic, castration-sensitive prostate cancer. N Engl J Med 2019 Jul 4;381(1):13-24.
- Armstrong AJ, Szmulewitz RZ, Petrylak DP, et al. ARCHES: A Randomized, Phase III Study of Androgen Deprivation Therapy with Enzalutamide or Placebo in Men with Metastatic Hormone-Sensitive Prostate Cancer. J Clin Oncol. 2019 Nov 10;37(32):2974-2986.
- Davis ID, Martin AJ, Stockler MR, et al. Enzalutamide with Standard First-Line Therapy in Metastatic Prostate Cancer. N Engl J Med 2019 Jul 11;381(2):121-131.
- Lowentritt B, Pilon D, Waters D, et al. Comparison of prostate-specific antigen response in patients with metastatic castration-sensitive prostate cancer initiated on apalutamide or abiraterone acetate: A retrospective cohort study. Urol Oncol. 2023 May;41(5):252.e19-252.


