(UroToday.com) The 2024 GU ASCO annual meeting featured a prostate cancer session and a presentation by Dr. Praful Ravi discussing patient outcomes after therapy with 177Lu-PSMA-617 (LuPSMA) for metastatic castrate-resistant prostate cancer (mCRPC).
While LuPSMA has a proven overall survival benefit in patients with mCRPC who have received prior taxane chemotherapy and androgen receptor pathway inhibitors, there is limited data on outcomes and efficacy of subsequent therapy in patients who have received LuPSMA. This study presented at GU ASCO 2024 aimed at assessing the clinical and treatment courses of patients with mCRPC after LuPSMA.1
Dr. Ravi and colleagues queried an IRB-approved prospectively maintained registry to evaluate all patients with mCRPC who received standard-of-care LuPSMA at the Dana-Farber Cancer Institute between June 2022 and July 2023. Clinical data pertaining to LuPSMA therapy as well as subsequent treatments were extracted from the medical record, including type and number of subsequent systemic therapies, reason for treatment cessation, hematologic support, and PSA response to subsequent therapy. PSA-50 was defined as a ≥50% decrease in PSA on therapy. Hematologic adverse events to therapies after LuPSMA were recorded and graded per CTCAE v5.0.
A total of 96 patients were evaluated, with a median age of 72 years (range 52-87), and median follow-up of 10 months. 18 patients completed all 6 cycles, 35 discontinued prior to cycle 6 of LuPSMA due to progressive disease, 10 died mid-treatment, and 33 were midway through treatment. The median number of cycles received for all patients was 4 (range 1-6). None of the 18 who completed all 6 cycles had yet transitioned to a next line therapy. Of the 35 who discontinued LuPSMA, 17 transitioned to hospice due to disease progression, 15 initiated a subsequent line of therapy, and 3 had a treatment break. The patient disposition is as follows: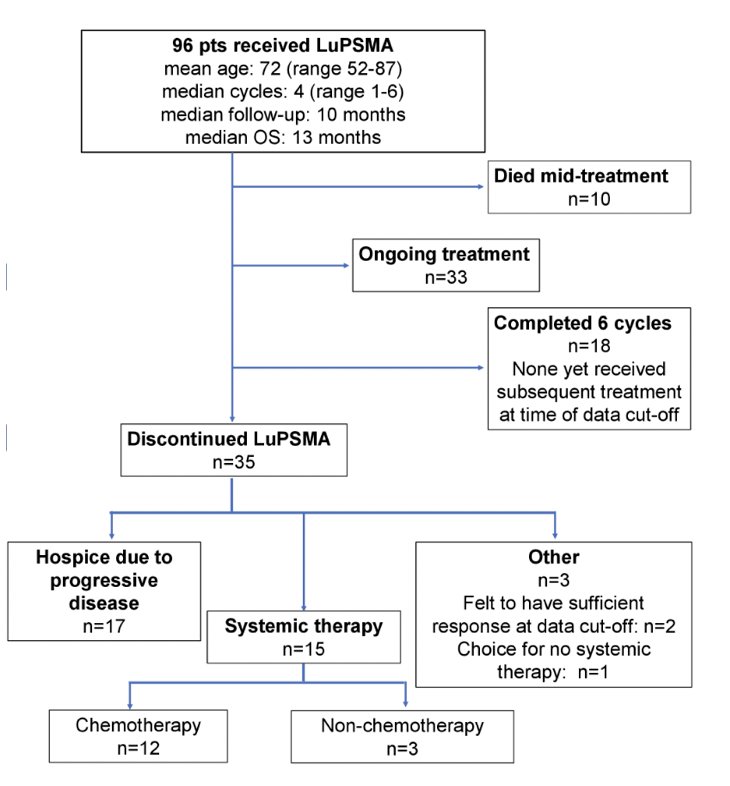
The median overall survival was 13 months in the entire cohort. Of the 15 receiving another line of therapy, 11 received cabazitaxel ± carboplatin/cisplatin, 1 received carboplatin with cisplatin substituted during carboplatin shortage, and 3 received other non-chemotherapy regimens. A PSA-50 response to subsequent therapy was seen in 4 patients (27%), all of whom received cabazitaxel ± carboplatin/cisplatin. Of the 12 patients who received chemotherapy, the median number of cycles received was 3 (range 1-7). There were 4 patients (33%) that had grade ≥3 anemia, 2 (17%) had grade ≥3 thrombocytopenia and 7 patients (47%) required platelet growth factor support or transfusion of blood products: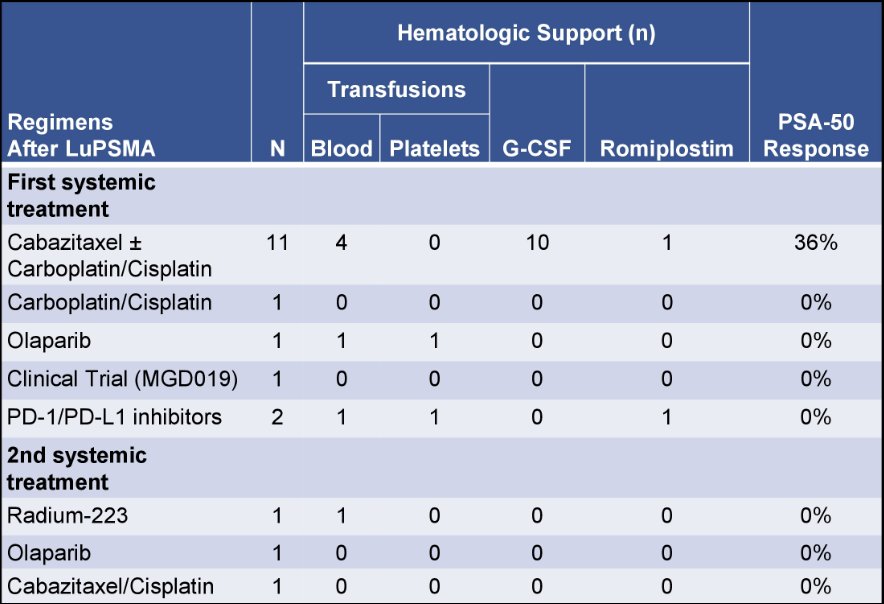
Dr. Ravi concluded his presentation discussing patient outcomes after therapy with 177Lu-PSMA-617 for mCRPC with the following take-home points:
- 47% of patients discontinuing LuPSMA transitioned to hospice due to disease progression while 44% proceeded to a subsequent line of therapy, the majority of whom received cabazitaxel
- Outcomes to subsequent therapy were generally poor, with a 27% PSA-50 response rate
- This initial experience may be biased by number of patients with limited standard of care options at the time of FDA approval of LuPSMA and could improve with better patient selection
- However, these results highlight the continued need to develop novel therapeutic strategies for mCRPC patients post-LuPSMA
Presented by: Praful Ravi, MB, BChir, MRCP, Dana-Farber Cancer Institute, Boston, MA
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the Genitourinary (GU) American Society of Clinical Oncology (ASCO) Annual Meeting, San Francisco, CA, Thurs, Jan 25 – Sat, Jan 27, 2024.
Reference:


