(UroToday.com) The 2024 GU ASCO annual meeting included a prostate cancer session featuring trials in progress and a presentation by Dr. Ulrich Krafft discussing the trial design of the NEPI trial, a randomized phase I/II study of neoadjuvant treatment with 177-Lutetium-PSMA-617 (LuPSMA) with or without ipilimumab in patients with very high-risk prostate cancer who are candidates for radical prostatectomy. High-risk prostate cancer accounts for approximately 15% of newly diagnosed prostate cancers, and for these patients with high-risk, locally advanced prostate cancer, prostatectomy alone may be insufficient therapy. Indeed, cure rates from radical prostatectomy alone are less than 25%.
Ipilimumab increases overall survival in men with post-docetaxel mCRPC who received radiotherapy to bone metastases followed by ipilimumab in comparison to placebo. Additionally, the final results of the VISION trial, a phase III study designed to assess the efficacy LuPSMA in patients with PSMA-positive mCRPC, show a significant prolongation of survival in a setting without established therapy.1 Since both ipilimumab combined with radiation and LuPSMA have been proven to be effective in the treatment of mCRPC, Dr. Krafft and colleagues hypothesized that CTLA-4-inhibitors exert synergistic effects on LuPSMA radioligand therapy-induced tumor damage and immune priming.
Patients diagnosed with highest risk prostate cancer (ISUP-GG 4+5 and cT3 plus cN+ or PSA > 20ng/ml) who are candidates for radical prostatectomy receive 12 weeks of neoadjuvant treatment with ADT + LuPSMA with randomized allocation with or without ipilimumab. Prior to initiation of the randomized set, the trial will conduct a safety-run-in-phase of an initial 3-6 patients that will receive a reduced dose of 3.7 Gb LuPSMA and 3 mg/kg ipilimumab, which will be increased to the target dose of 7.4Gb LuPSMA and 3 mg/kg ipilimumab in the following 3-6 patients if sufficiently well tolerated. The safety and feasibility of the combination will be assessed after 6-12 patients based on toxicity or delay of prostatectomy (maximum 3 weeks). In the absence of serious toxicity signals, a total of 46 patients will be randomized 1:1 (ipilimumab + ADT + LuPSMA vs. ADT + LuPSMA) to receive 2 cycles of 7.4 GBq LuPSMA with or without 4 cycles of ipilimumab 3mg/kg prior to prostatectomy:
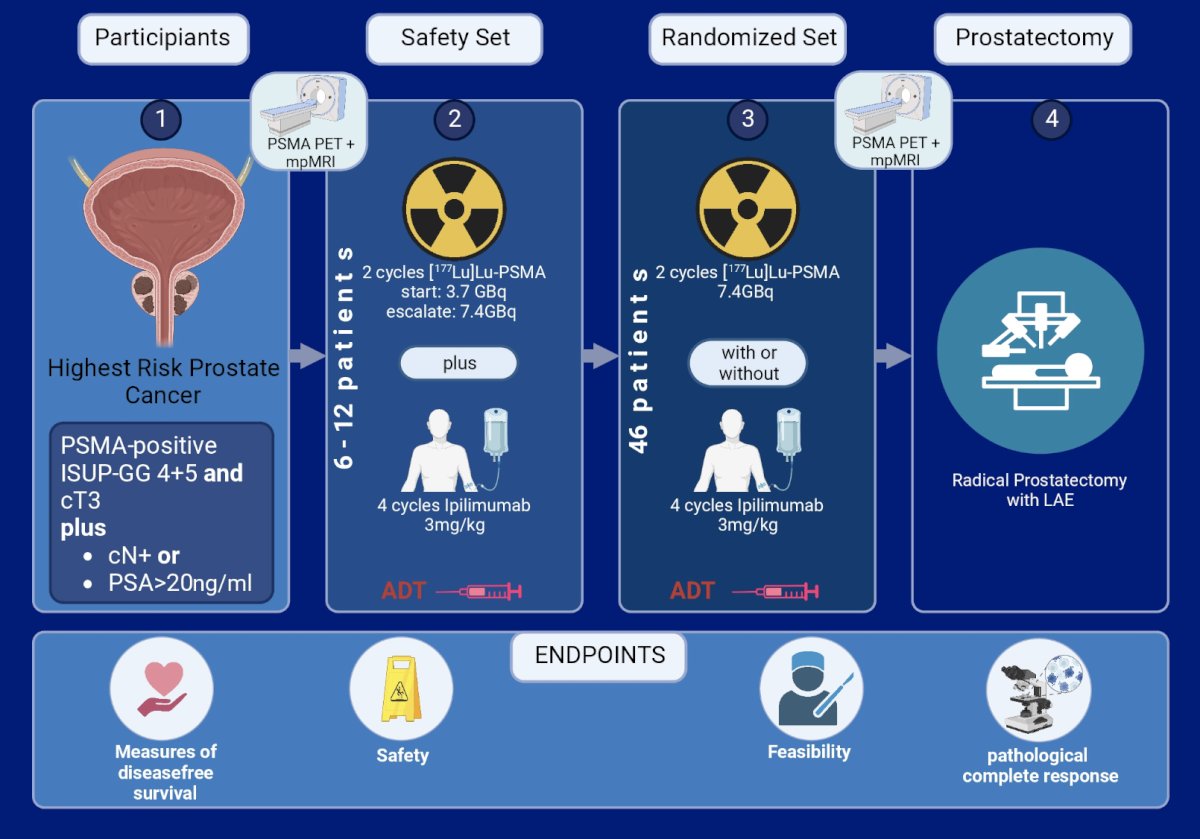
Scheduled imaging with mpMRI and PSMA PET/CT is performed prior to neoadjuvant therapy and prior to radical prostatectomy. The co-primary endpoints of the study are feasibility of radical prostatectomy after neoadjuvant therapy and complete pathologic response.
Secondary endpoints are:
- Evaluation of the safety profile of neoadjuvant treatment according to CTCAE v5.0
- Disease-free survival
Enrollment of the first patient in NEPI Trial is expected to be completed in late September 2023. A total four German centers will participate.
Clinical trial information: EudraCT-Nr.: 2021-004894-30.
Presented by: Ulrich Krafft, University Hospital Essen, University Duisburg-Essen, Essen, Germany
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, San Francisco, CA, January 25th – January 27th, 2024
References:


