(UroToday.com) The 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) cancers symposium held in San Francisco, CA between January 25th and 27th was host to a urothelial carcinoma poster session. Ian McElree presented the results of a study comparing sequential endoluminal gemcitabine plus docetaxel versus bacillus Calmette-Guerin (BCG) for the treatment of high-grade upper tract urothelial carcinoma (UTUC).
Currently, BCG remains the only approved endoluminal treatment option for the management of high-grade UTUC (Jelmyto® approved for low-grade tumors). The combination of endoluminal gemcitabine + docetaxel has shown promising efficacy for the management of high-grade UTUC, although a direct comparison of this combination with BCG remains unavailable. Accordingly, the objective of this study was to compare the outcomes of high-grade UTUC patients receiving endoluminal gemcitabine/cisplatin to BCG.
This was a retrospective analysis of 59 patients, with 71 upper tract tumors, of which 35 and 36 were treated with gemcitabine/docetaxel and BCG, respectively. Patients in the gemcitabine/docetaxel group received six weekly induction instillations of 1 gm gemcitabine + 37.5 mg docetaxel instilled via a percutaneous nephrostomy tube or a retrograde ureteral catheter. Patients in the BCG group received 1 vial of BCG (+/- IFNa-2b) at a similar frequency via the same approach. Maintenance therapy was recommended if patients had no evidence of disease recurrence. The primary outcome was recurrence-free survival. Survival analyses using Kaplan Meier estimates and Cox regression modeling were performed.
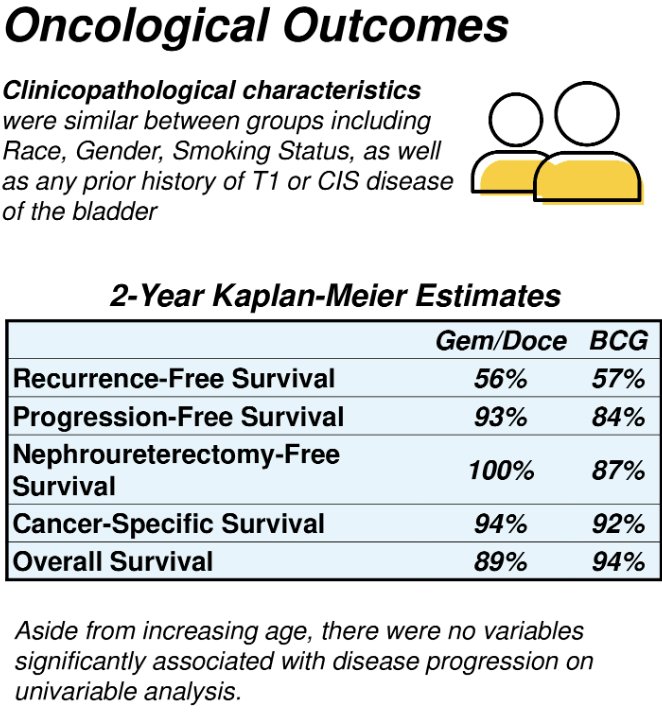
The median follow-up for BCG-treated patients was 62 months and 29 months for gemcitabine/docetaxel. The 2-year recurrence-free survival was similar in the two treatment groups: 57% for BCG and 56% for gemcitabine/docetaxel.
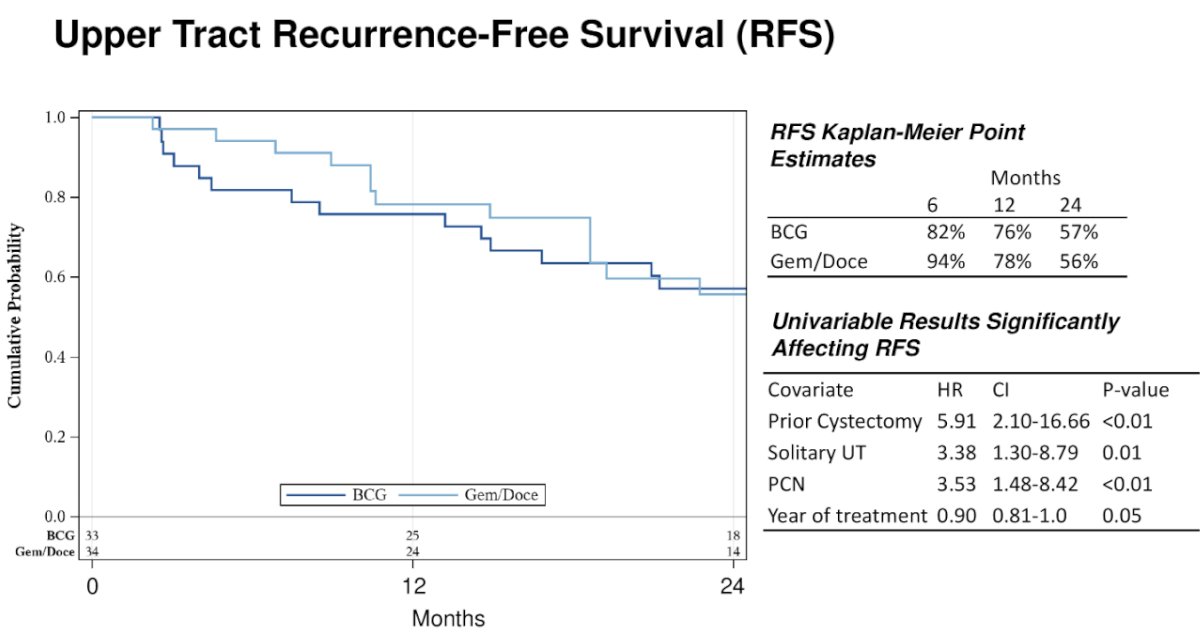
The nephroureterectomy-free survival at 2 years was 87% with BCG and 100% with gemcitabine/docetaxel.
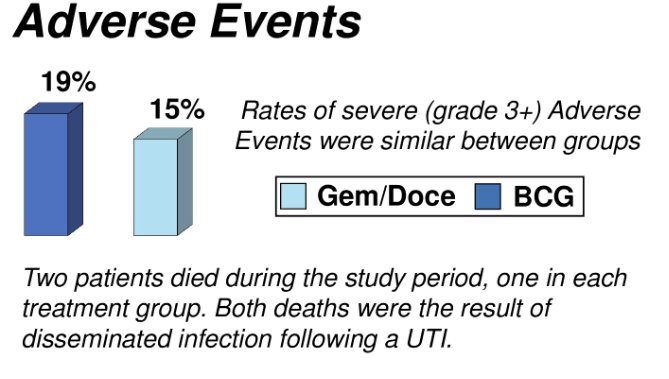
There were similar rates of adverse events (p=0.3).
The investigators concluded that endoluminal gemcitabine/docetaxel and BCG have similar oncological outcomes and major adverse event rates for the treatment of high-grade UTUC. Further prospective evaluation is warranted based on these retrospective findings.
Presented by: Ian M. McElree, Medical Student, The University of Iowa Carver College of Medicine, Iowa City, Iowa City, IA
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2024 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, San Francisco, CA, January 25th – January 27th, 2024


