The SAVOIR study was an open-label, randomized study, among patients with centrally confirmed MET-driven (MET and/or HGF amplification, chromosome 7 gain and/or MET kinase domain mutations), metastatic papillary renal cell carcinoma (RCC) who were randomized to savolitinib 600 mg once daily, or sunitinib 50 mg daily for 4 weeks on/2 weeks off. The primary objective of this trial was progression-free survival by RECIST 1.1 by blinded independent central review, and secondary objectives included overall survival, objective response rate (ORR), and safety and tolerability. Initial results of this trial were recently presented by Dr. Toni Choueiri at ASCO 2020. Only 60 of the planned 180 patients were randomized (savolitinib n = 33; sunitinib n = 27), although progression-free survival, overall survival, and ORR were numerically improved with savolitinib vs sunitinib:
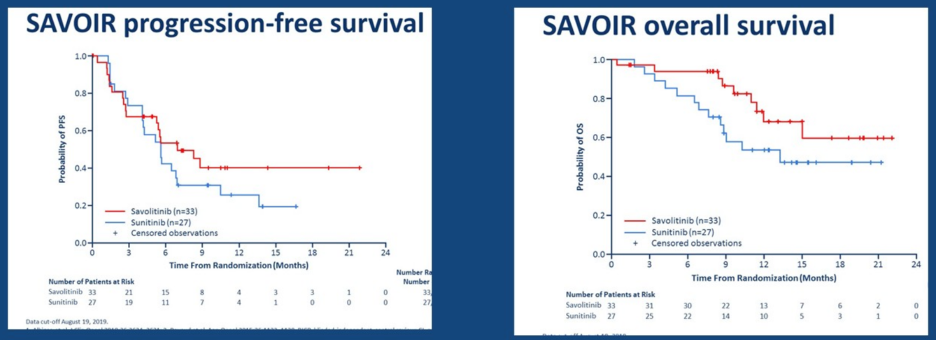 Dr. McGregor noted that the objective response rate for those receiving savolitinib was 27% compared to 7% for sunitinib. So, although these results are encouraging for papillary RCC, it is less clear with clear-cell RCC. Dr. McGregor postulates that this may be secondary to a low prevalence of actionable mutations in RCC compared to other solid tumors. Currently, biomarkers/clinical predictors are as follows:
Dr. McGregor noted that the objective response rate for those receiving savolitinib was 27% compared to 7% for sunitinib. So, although these results are encouraging for papillary RCC, it is less clear with clear-cell RCC. Dr. McGregor postulates that this may be secondary to a low prevalence of actionable mutations in RCC compared to other solid tumors. Currently, biomarkers/clinical predictors are as follows:
- Clinical applications: IMDC risk classification, neutrophil-lymphocyte ratio, PD-L1 staining, and sarcomatoid and rhabdoid histology
- Genomics: next-generation sequencing (assessing tumor mutational burden and PBRM1 status), genomic signatures, and metabolomics
In the age of VEGF inhibitors, we primarily relied on the IMDC model, taking into account Karnofsky performance status <80, time to initiation of targeted therapy <1 year, hemoglobin < lower level of normal and other laboratory values (serum calcium, neutrophil count, and platelet count) > upper limit of normal. Results from CheckMate 2141 noted no difference in outcomes between nivolumab plus ipilimumab versus sunitinib for patients with IMDC favorable-risk disease, whereas KEYNOTE-4262 noted a trend towards improved outcomes for patients receiving pembrolizumab plus axitinib versus sunitinib in these favorable-risk patients. However, Dr. McGregor cautions that long-term follow-up is critical to determine the predictive role of IMDC risk classification in the era of immunotherapy.
The neutrophil-lymphocyte ratio showed decent prognostic capabilities among patients receiving targeted therapy, whereby higher neutrophil-lymphocyte ratio at baseline was associated with shorter overall survival and progression-free survival (HR per 1 unit increase in log-transformed neutrophil-lymphocyte ratio = 1.69, 95% CI 1.46-1.95 and 1.30, 95% CI 1.15-1.48, respectively).3 Initial results assessing neutrophil-lymphocyte ratio for patients treated with immunotherapy suggests that an early decline in neutrophil-lymphocyte ratio at 6 weeks of treatment may be associated with improved outcomes.
PD-L1 has historically been a poor prognostic marker in the VEGF era. There are many limitations of PD-L1 as highlighted by Dr. McGregor in the following table summary: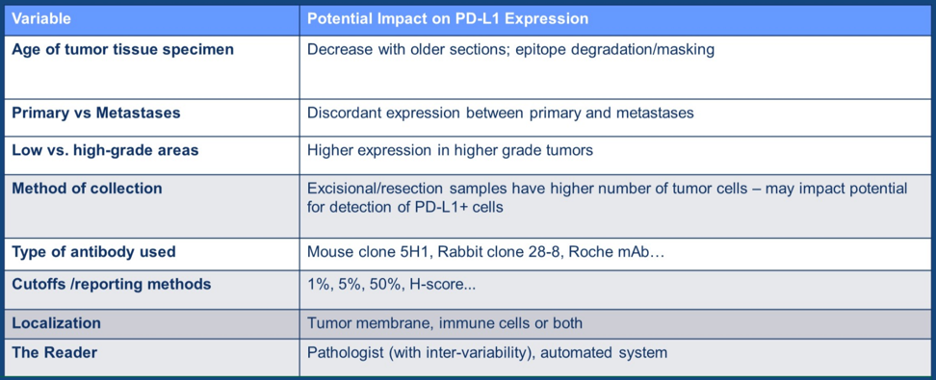
However, PD-L1 status was prognostic for second-line treatment with nivolumab in the CheckMate-025 study of nivolumab versus everolimus.4 Among patients with 1% or greater PD-L1 expression, the median overall survival was 21.8 months (95% CI 16.5-28.1) in the nivolumab group and 18.8 months (95% CI 11.9-19.9) in the everolimus group (HR 0.79, 95% CI 0.53-1.17). Furthermore, among patients with less than 1% PD-L1 expression, the median overall survival was 27.4 months (95% CI 21.4 to not estimable) in the nivolumab group and 21.2 months (95% CI 17.7-26.2) in the everolimus group (HR 0.77, 95% CI 0.60-0.97). Although the data is still somewhat immature, PD-L1 status suggests there may be prognostic evidence among patients treated with combination therapy in the CheckMate 214 and KEYNOTE-426 trials.
Not surprisingly, patients with sarcomatoid differentiation typically do quite poorly. Data from the prominent phase III trials does suggest that patients with sarcomatoid differentiation treated with combination therapy do better than those treated with sunitinib alone: 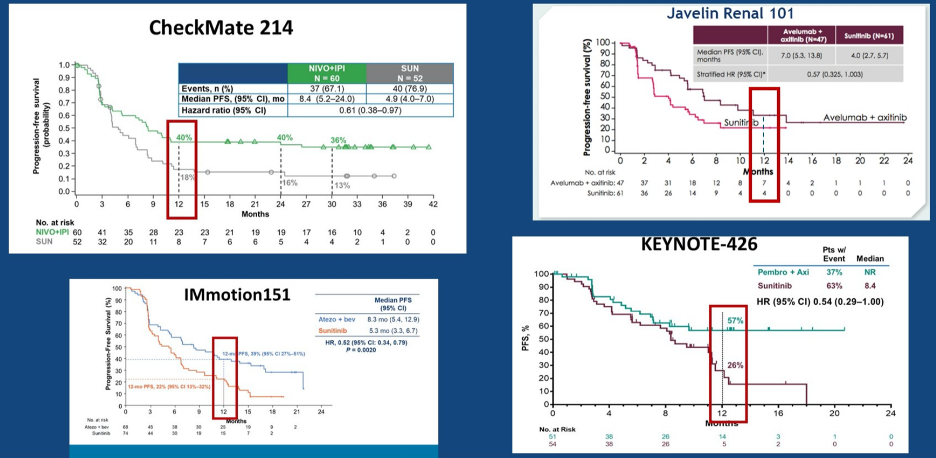
Switching to genomic biomarkers, Dr. McGregor notes that data from the COMPARZ5 and RECORD36 trials suggests that PBRM1 loss may be associated with improved outcomes. However, the story is more complicated when assessing this biomarker in patients treated with nivolumab, as there is no association with response to nivolumab. Gene signatures from ImMotion 150 suggest that tumor mutation and neoantigen burden were not associated with progression-free survival, however angiogenesis, T-effector/IFN-γ response, and myeloid inflammatory gene expression signatures were strongly and differentially associated with progression-free survival within and across treatment.
Javelin RENAL 101 assessed avelumab plus axitinib versus sunitinib, demonstrating superior outcomes of the combination therapy.8 Additionally, the investigators also developed a 26-gene Javelin RENAL 101 signature, with data initially presented by Dr. Choueiri at the ASCO 2019 annual meeting. Whole transcriptome data from 720 baseline tumor samples were filtered for informative genes, followed by individuals blinded to the outcome assessed co-expression to identify 306 genes. High expression of a 306-gene signature was associated with better PFS in the avelumab plus axitinib arm but not in the sunitinib arm. Further filtering of the co-expressed 306 genes based on the immune-related functionality and most significant association with progression-free survival in the avelumab plus axitinib arm identified the 26-gene subset. Progression-free survival according to the signature showed that high expression in the avelumab plus axitinib arm lead to a PFS benefit (HR 0.60, 95%CI 0.439-0.834), but no difference in the sunitinib arm (HR 0.89, 95%CI 0.670-1.172).
Metabolomics have also been assessed as potential biomarkers for RCC. Based on data presented at ASCO 2017, patients with low adenosine had improved median progression-free survival (median 5.4 months) compared to those with high adenosine (median 2.5 months; p=0.0037). Additionally, the kynurenine/tryptophan ratio has also been explored, whereby increased tryptophan to kynurenine inhibits T cell proliferation. By suppressing this pathway, tumor immune resistance could be reversed. As follows is a summary table of the current state of biomarkers/clinical predictors:
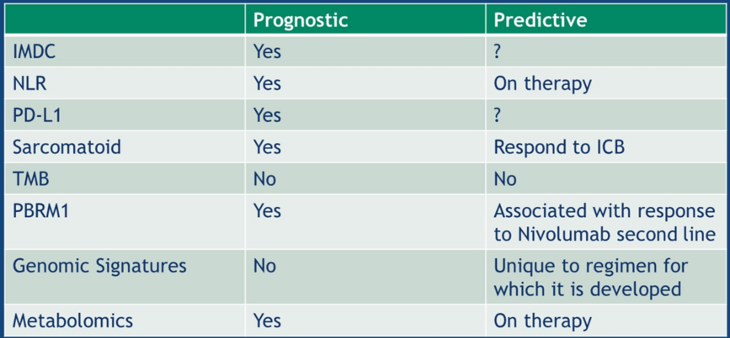
In conclusion, Dr. McGregor provided the following take-home messages:
- There are multiple prognostic markers
- What works for other tumors does not work in RCC: tumor mutational burden, PD-L1, MSI
- There are exciting “potential” predictive biomarkers: genomics (not tumor mutational burden), metabolomics
- One size fits all does not fit for RCC: biomarkers are not ready for prime time in RCC in August 2020
Presented by: Bradley A. McGregor, MD, Dana-Farber Cancer Institute, Boston, MA
Written by: Zachary Klaassen, MD, MSc – Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, Twitter: @zklaassen_md at the ASCO20 Virtual Education Program, #ASCO20, August 8-10, 2020.
References:
- Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus Ipilimumab versus Sunitinib in Advanced Renal-Cell Carcinoma. N Engl J Med 2018;378(14):1277-1290.
- Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus Axitinib versus Sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med 2019;380(12):1116-1127.
- Templeton AJ, Knox JJ, Lin X, et al. Change in Neutrophil-to-lymphocyte Ratio in Response to Targeted Therapy for Metastatic Renal Cell Carcinoma as a Prognosticator and Biomarker of Efficacy. Eur Urol 2016 Aug;70(2):358-364.
- Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med 2015;373(19):1803-1813.
- Motzer RJ, Hutson TE, Cella D, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med 2013;369(8):722-731.
- Hsieh JJ, Chen D, Wang PI, et al. Genomic Biomarkers of a Randomized Trial Comparing First-line Everolimus and Sunitinib in Patients with Metastatic Renal Cell Carcinoma. Eur Urol 2017 Mar;71(3):405-414.
- McDermott DF, Huseni MA, Atkins MB, et al. Clinical activity and molecular correlates of response to atezolizumab alone or in combination with bevacizumab versus sunitinib in renal cell carcinoma. Nat Med. 2018 Jun;24(6):749-757.
- Motzer RJ, Penkov K, Haanen J, et al. Avelumab plus axitinib versus sunitinib for Advanced Renal-Cell Carcinoma. N Engl J Med 2019;380(12):1103-1115.


