(UroToday.com) The Latin American sectional meeting at the 2021 American Society for Radiation Oncology annual meeting included a presentation by Dr. Christian Flores Balcazar discussing updates and controversies in the NCCN guidelines for radiation therapy and prostate cancer. Dr. Flores Balcazar started by highlighting that over the years the NCCN has become the market leader for clinical guidelines, reuniting the experts often to summarize the diagnosis and management options currently available. As a result, this document is quickly updated, is easy to consult for patients and clinicians, and is free of charge to anyone. However, the main drawback does not have to do with the content itself, but rather how these guidelines are used. Multidisciplinary participation in the elaboration of the NCCN prostate cancer guidelines is crucial, and this includes medical oncologists, urologists, radiation oncologists, and patient advocates. As follows, is the breakdown by specialty among the experts:

Dr. Flores Balcazar notes that there are several key points with regards to what is new in version 1.2022, including (i) technology is modified to be more inclusive, (ii) the syntax is extensively revised, (iii) there is a section called “Principles of Risk Stratification,” and (iv) there is a section called “Non-hormonal Systemic Therapy for Very-High-Risk Prostate Cancer.”
Post-prostatectomy radiation therapy had several updates in the 1.2022 new guidelines. For PROS-3 to PROS-7 discussing adjuvant/salvage EBRT for very-low to very-high-risk groups, the word “observation” was changed to “monitoring” and radiotherapy as an option, highlighted as follows:

There is evidence supporting EBRT for persistently elevated PSA after radical prostatectomy, including the RTOG 9601 trial, ARO 96-02, EORTC 22911, and SWOG S8794. However, there is also evidence supporting monitoring and early salvage EBRT. In the ARTISTIC planned systematic review of prospective clinical trials, based on the RAVES, RADICALS-RT trials, and the GETUG-AFU 17 trial, the ARTISTIC collaboration was a preplanned, prospective effort to undertake meta-analysis of each of the three trials comparing adjuvant and early salvage radiotherapy.1 Across the three trials, a total of 1,074 men were randomized to adjuvant radiotherapy and 1,077 to an early salvage strategy. Despite some differences in patient population and study design, the findings of the three trials were remarkably similar: there was no significant improvement in biochemical event free survival for patients receiving adjuvant radiotherapy (hazard ratio 1.12, 95% confidence interval 0.88 to 1.42). Further, among patients randomized to an early salvage strategy, 395 (37%) have thus far commenced salvage radiotherapy and the remainder have been spared therapy.
With regards to the addition of ADT to post-prostatectomy EBRT, NCCN guidelines version 2.2021 was supported by results of the RTOG 9601 and GETUG-16 trials showing a benefit of short term ADT. For version 1.2022, this portion of the guidelines was also supported by preliminary results of the SPPORT Trial:

With regards to definitions of radiation therapy, the updated NCCN guidelines for brachytherapy added an additional bullet stating:
- Interstitial implantation of prostate +/- proximal seminal vesicles with temporary (high dose-rate, HDR) or permanent (low dose-rate, LDR) radioactive sources for monotherapy or as “boost”, when added to EBRT, should be performed in practices with adequate training, experience, and quality assurance measures.
The NCCN guidelines state that patient selection should consider aspects of gland size, baseline urinary symptoms, and prior procedures (ie. TURP) that may increase risk of adverse effects. Neoadjuvant ADT to shrink the gland to allow treatment should balance its additional toxicity with this benefit. To this statement, the following bullet point has been added to the 1.2022 updated guideline:
- Post-implant dosimetry must be performed for brachytherapy boost when added to EBRT and ADT, which improves biochemical control. To address historical trial data concern for increased toxicity incidence, careful patient selection, and contemporary planning is associated with lesser toxicity, such as the use of recognized organ at risk dose constraints, use of high-quality ultrasound and other imaging, and prescription of dose as tightly as possible to the target without excessive margins.
In the section of the guidelines discussing EBRT for very-high-risk prostate cancer, abiraterone and docetaxel were added to EBRT + ADT as an option for management:

There is evidence supporting abiraterone + ADT and EBRT in very-high-risk groups, presented recently at the ESMO 2021 virtual annual meeting. This included the combined analysis from two comparisons in the STAMPEDE platform assessing standard of care (radiotherapy plus 3 years of ADT) to standard of care plus abiraterone acetate + prednisolone with or without enzalutamide for two years. With six years of median follow-up, there was a 47% reduction in metastasis free survival for the addition of abiraterone acetate + prednisolone with or without enzalutamide to standard of care (p = 2.9 x10^-11). This translated to six-year metastasis free survival of 82% on the standard of care plus abiraterone acetate + prednisolone with or without enzalutamide compared to 69% on the standard of care arm:

Additionally, with six years of median follow-up, there was a 40% reduction in OS for the addition of abiraterone acetate + prednisolone with or without enzalutamide to standard of care (p = 9.3 x10^-7). This translated to six-year OS of 86% on the standard of care plus abiraterone acetate + prednisolone with or without enzalutamide compared to 77% on the standard of care arm. As seen with metastasis free survival, there was no significant difference in OS across randomization periods, further supporting no additional improvement with the addition of enzalutamide to abiraterone acetate + prednisolone:

Evidence supporting docetaxel + ADT and EBRT in very-high risk groups is provided by the STAMPEDE docetaxel data2 which showed that high-risk M0 patients (in addition to the M1 patients) benefited from docetaxel. This did not confer a survival benefit for M0 patients (HR 0.95, 95% CI 0.62-1.47), however, a significant failure-free survival benefit of 9 months was noted.
With regards to regional risk-groups for the management of any T, N1, M0 patients, the updated guidelines removed from the title “regional and metastatic risk group”, as well as added surgery as a management option:
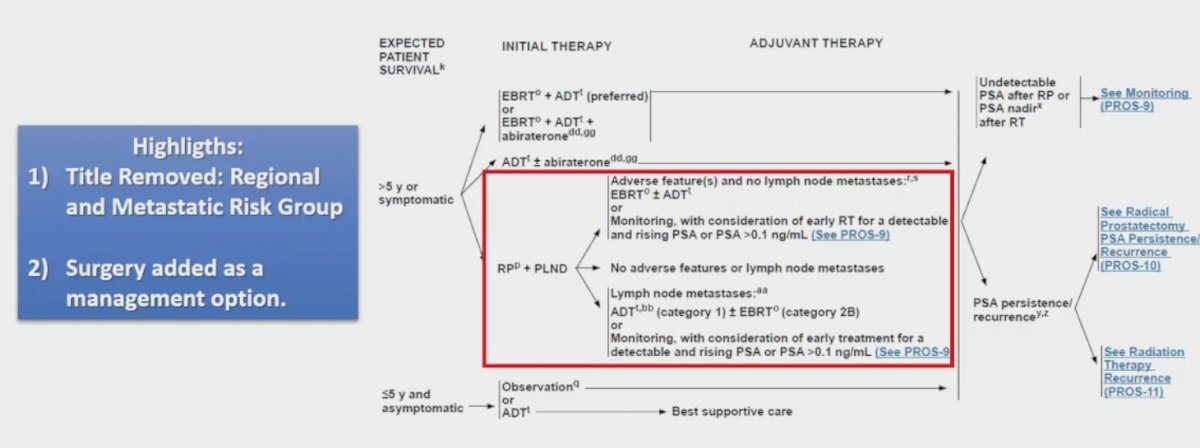
Evidence supporting surgery in patients with clinical N1 disease is based on retrospective data, specifically a study assessing survival among 741 men with cN+ disease who underwent radical prostatectomy and were compared to those who received ADT + radiotherapy and ADT alone.3 This study found that there was no significant difference in risk of prostate cancer specific mortality (p=0.13) or all-cause mortality (p=0.16).
Dr. Flores Balcazar concluded this presentation of updates from the 1.2022 version of the NCCN guidelines with the following take-home points:
- The NCCN guidelines are intended to help clinicians in the decision-making process of prostate cancer management
- The document lays out principles that should be followed to evaluate and counsel patients in management
- However, this and all guidelines should be addressed with caution because the “one size fits all” principle does not apply in oncology
- For radiotherapy, its role in all stages of prostate cancer is undeniable
- The increasing accuracy of treatments allows for more abbreviated and ablative treatments for a better quality of life
- A new and increasing role in the oligometastatic setting is ongoing
- If the recent trend for surgeons to take on locally advanced disease continues, the role of salvage EBRT as part of a planned multimodal approach will grow
- The next decade will continue to see radiation oncology evolve as novel biomarkers will help stratify and personalize treatment recommendations
Presented by: Christian Flores Balcazar, Department of Radiotherapy, Instituto Nacional de Ciencias Medicas y Nutricion Salvador Zubiran, Mexico City, Mexico
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2021 American Society for Radiation Oncology (ASTRO) Hybrid Annual Meeting, Sat, Oct 23 – Wed, Oct 27, 2021.
References:
- Vale CL, Fisher D, Kneebone A, et al. Adjuvant or early salvage radiotherapy for the treatment of localized and locally advanced prostate cancer: A prospectively planned systematic review and meta-analysis of aggregate data. Lancet 2020 Oct 31;396(10260):1422-1431.
- James ND, Sydes MR, Clarke NW, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387(10024):1163-1177.
- Gandaglia G, Soligo, Battaglia A, et al. Which patients with clinically node-posiitve prostate cancer should be considered for radical prostatectomy as part of multimodal treatment? The impact of nodal burden on long-term outcomes. Eur Urol. 2019 May;75(5):817-825.


