(UroToday.com) The 2021 American Society for Radiation Oncology (ASTRO) Hybrid Annual Meeting’s included a session on biomarkers and salvage radiotherapy and discussion by Dr. Liat Hammer regarding results of a phase 1 trail of neoadjuvant stereotactic body radiotherapy prior to radical prostatectomy in locally advanced prostate cancer.
Recurrence after radical prostatectomy for high-risk prostate cancer is common, with ~80% of men recurring or requiring post-operative therapy after surgery alone. Recent work has suggested that there is increasing use of surgery for high risk prostate cancer, however with lower utilization of salvage radiotherapy, including ~70% of men with biochemical recurrence not receiving salvage radiotherapy. The aim of the current study presented at ASTRO 2021 by Dr. Hammer and colleagues was to determine if neoadjuvant stereotactic body radiotherapy followed by radical prostatectomy (without ADT) would be safe and effective in high-risk and clinically node positive prostate cancer.
Neoadjuvant radiotherapy for cancer treatment is not uncommon and is often utilized for esophageal cancer, NSCLC, rectal cancer, breast cancer, and sarcoma. There are several pros to neoadjuvant radiotherapy, including a smaller volume of radiotherapy, lower dose, shorter treatment courses, improved compliance, and surgical removal of radiated tissues. With regards to cons of a neoadjuvant radiotherapy approach, this includes suboptimal surgical healing and unnecessarily treating patients who may not recur.
The primary objective of this phase I study was to determine the maximum tolerated dose of neoadjuvant stereotactic body radiotherapy that leads to <=28% of patients experiencing a dose-limiting toxicity 30 days post-radical prostatectomy, defined as any of the following:
- Blood loss > 400 mL
- Hospital stay > 2 days
- Drain placement > 2 days
- Catheter placement > 16 days
- Indwelling catheter replacement
- Readmission
- Death
Secondary objectives included assessing adverse events (GI, genitourinary, and sexual), efficacy (biochemical recurrence, rate of distant metastases), and multiple quality of life inventories (IPSS, IIEF, EPIC-26). The study was statistically designed to enroll 38 evaluable subjects. Inclusion criteria included: (i) adenocarcinoma histology, (ii) PSA >= 20 ng/mL, (iii) Grade group 4-5, (iv) stage >= T3a, (v) N1 disease, (vi) several intermediate risk factors, and (vii) Karnofsky performance status >= 70. Key exclusion criteria included (i) the patient being unable to undergo surgery, (ii) metastatic spread of disease, (iii) prior pelvic radiotherapy, and (iv) prior ADT (last 3 months). The trial schema is as follows:

This trial was ultimately stopped early due to unacceptably high toxicity following radical prostatectomy, although the primary endpoint was not reached. Among 16 patients accrued to the trial, the following are the baseline clinicopathologic characteristics:

Median follow-up was 40 months (IQR 33-44) and stereotactic body radiotherapy of 35 Gy/5 fractions was delivered in 14 patients, whereas 30 Gy/5 fractions was delivered in 2 patients. There were several severe complications including grade 3 urinary tract obstruction in 25% of patients, grade 3 urinary fistula in 25% of patients, two patients (13%) requiring a cystectomy and urinary diversion, and two patients (13%) undergoing incision of bladder neck contracture. The results of urinary quality of life included ~75% of patients requiring at least one pad per day after surgery at 24 months and are as follows:

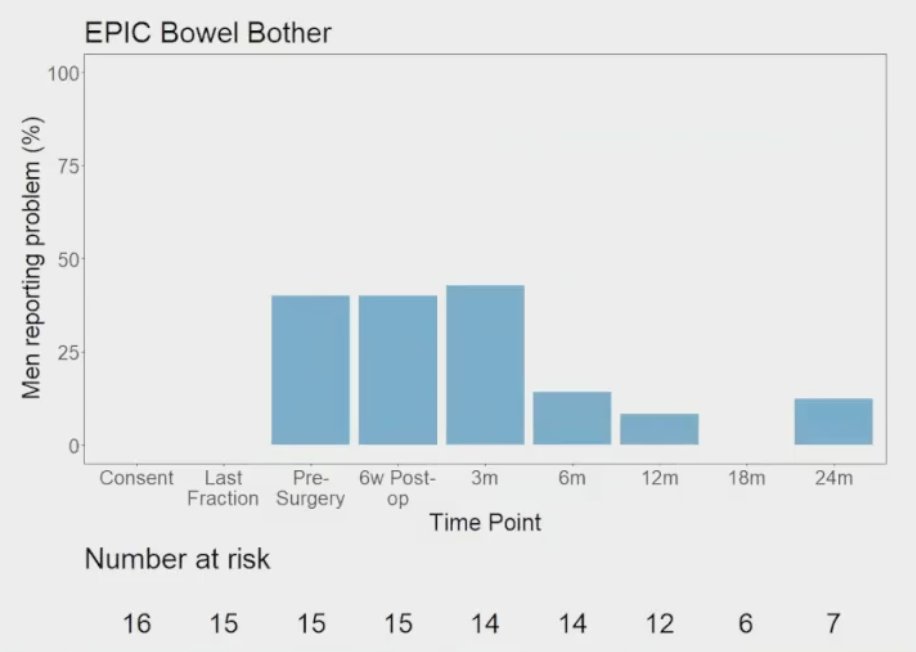
The results of bowel quality of life are as follows:
The results of erectile dysfunction quality of life include 100% of men reporting erectile dysfunction at 6 weeks post-op all the way through 24 months of follow-up and are as follows:

With regards to tumor control outcomes, surgical margins were negative for all patients and no patients developed a local or regional recurrence.
Dr. Hammer concluded her presentation with the following key take-home messages:
- These results do not support neoadjuvant stereotactic body radiotherapy followed by radical prostatectomy in the management of locally advanced prostate cancer
- Neoadjuvant stereotactic body radiotherapy followed by radical prostatectomy for the treatment of locally advanced prostate cancer resulted in unacceptably high toxicity and several quality of life declines
- The late toxicity highlights the importance of continued follow-up, even for phase 1 trials
Presented by: Liat Hammer, MD, PhD, Department of Radiation Oncology, University of Michigan, Ann Arbor, MI
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2021 American Society for Radiation Oncology (ASTRO) Hybrid Annual Meeting, Sat, Oct 23 – Wed, Oct 27, 2021.


