(UroToday.com) The 2022 ASTRO annual meeting featured a session on considerations for treatment of PSMA-staged high-risk patients, including a presentation by Dr. Leslie Ballas discussing treatment of local regional disease in the era of PSMA PET. Dr. Ballas notes that the core of the issue is accurate assessment of the extent of disease (ie. metastatic vs localized prostate cancer) being essential for guiding treatment decisions. The proPSMA study was a multi-center, two-arm randomized controlled trial of men with histologically confirmed prostate cancer who were being considered for curative intent radical prostatectomy or radiotherapy.1 To be eligible for inclusion, men must have had at least one high-risk factor including PSA ≥ 20 ng/mL, ISUP grade group 3-5, or clinical stage T3 or greater. Following enrollment, patients were randomly assigned in a 1:1 ratio to either conventional imaging consisting of bone scan and CT or 68Ga-PSMA-11 PET/CT. Between 2017 and 2018, 302 patients were randomized to either conventional imaging (n = 152) or 68Ga-PSMA-11 PET/CT (n = 150). PSMA PET/CT had a 27% absolute greater AUC for accuracy compared to conventional imaging (95% CI for difference: 23 – 31%): 92% (95% CI: 88 – 95%) vs. 65% (95% CI: 60 – 69%). Imaging findings led to changes in management (treatment intent, modality, or delivery) in 28% of men in the PSMA PET/CT arm versus 15% of men in the conventional imaging arm.
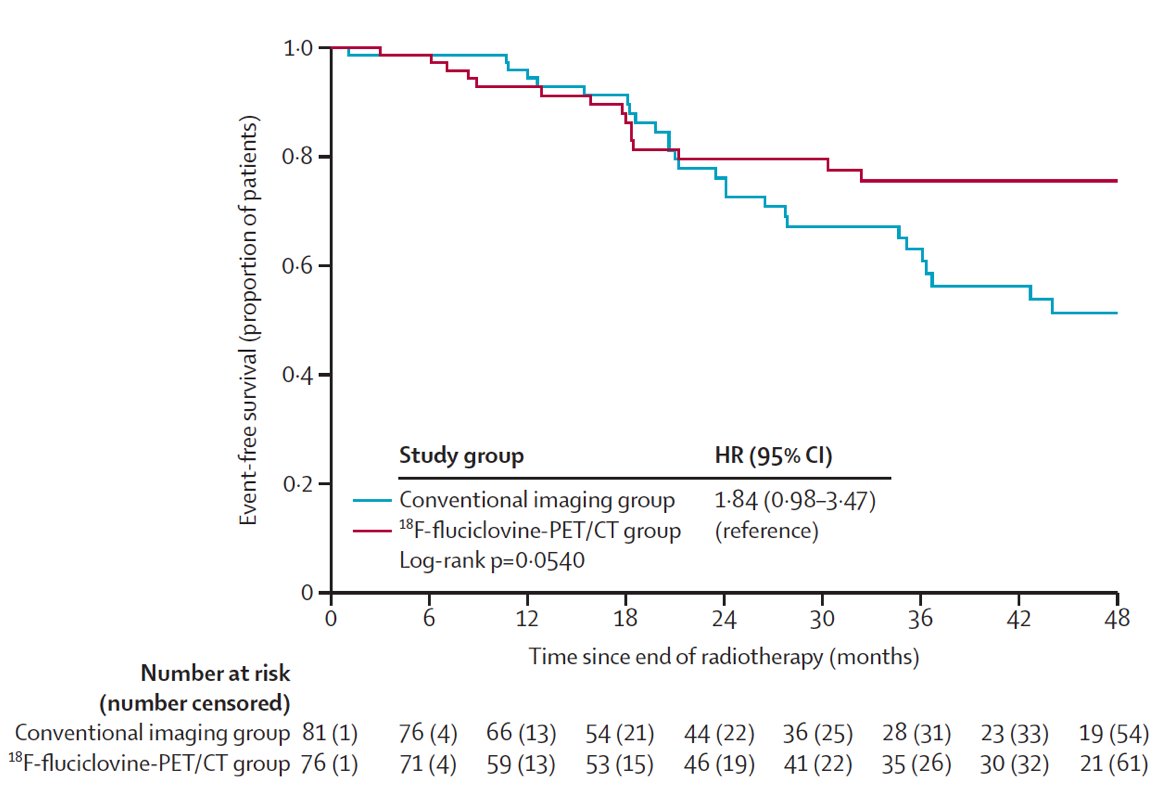
Despite these encouraging findings, does PET change outcomes? The EMPIRE-1 trial sought to evaluate whether 18F-fluciclovine improves cancer control compared to conventional imaging (bone scan + CT/MRI) alone for salvage post-prostatectomy radiotherapy.2 This was a single center, open label, phase 2/3 trial of post-prostatectomy patients with a detectable post-operative PSA and negative conventional imaging. These patients were randomly assigned in a 1:1 ratio to radiotherapy directed by conventional imaging alone or to conventional imaging plus 18F-fluciclovine-PET/CT. In the 18F-fluciclovine-PET/CT group, radiotherapy decisions were rigidly determined by PET findings, which were also used for target delineation. Between 2012 and 2019, this trial recruited 165 patients, with a median follow-up of 3.52 years. The 3-year event-free survival was significantly improved in the 18F-fluciclovine arm: 75.5% versus 63.0% (difference 12.5; 95% CI: 4.3–20.8; p = 0.0028):
Dr. Ballas wonders, now what? We have tools, but how do we use this information? There are several approaches for use criteria/appropriate use scenarios when utilizing PSMA PET/CT:
- Newly diagnosed unfavorable intermediate, high risk or very high risk prostate cancer
- The NCCN panel decided to include unfavorable intermediate risk along with high risk and very high risk categories in relation to the relevance of PSMA PET/CT
- A decision tree analysis of the cost per accurate diagnosis demonstrated that PSMA PET/CT was also less costly than conventional imaging for detection of disease spread
- Newly diagnosed unfavorable intermediate, high risk or very high risk prostate cancer with negative/equivocal or oligometastatic disease on conventional imaging
- PSA persistence or PSA rise from undetectable level after radical prostatectomy
- PSA rise above nadir after definitive radiotherapy
- Non-metastatic CRPC (M0) on conventional imaging
Dr. Ballas then discussed three key clinical trials that are incorporating PSMA PET/CT in the locally advanced disease space. The NRG-GU 008 trial requires PET imaging and has 3 options for radiation therapy schema:
- Standard fractionation with optional sequential boost: if molecular imaging showed visible pelvic disease (nodal), allow boost to visible disease to 54.0 – 63.0 Gy as organ at risk constraints allow (boost to visible disease is not required)
- Standard fractionation with optional simultaneous integrated boost: during the initial course treating the bilateral pelvis and prostate bed, simultaneous integrated boost to PET-positive pelvic areas is allowed where these areas may receive a total of 54.0 – 63.0 Gy (boost to visible disease is not required)
- Hypofractionation: if molecular imaging showed visible pelvic disease (nodal), allow either sequential or simultaneous integrated boost to visible disease to 54.0 – 63.0 Gy as organs at risk constraints allow (boost to visible disease is not required).
The trial schema of NRG-GU 008 is as follows:

NRG-GU 009 includes patients with confirmed N1 metastases on conventional imaging (CT/MRI) as defined by >10 mm on short axis, but will be automatically assigned to the intensification study. Patients who are positive by fluciclovine, choline or PSMA PET (ie. N1), but whose nodes do not meet traditional size criteria for positivity (ie they measure <= 10 mm on either the CT or MRI portion of the PET or on a dedicated CT or MRI) will not be considered N1 for the trial and will not automatically be assigned to the intensification study. The trial schema for NRG-GU 009 is as follows:

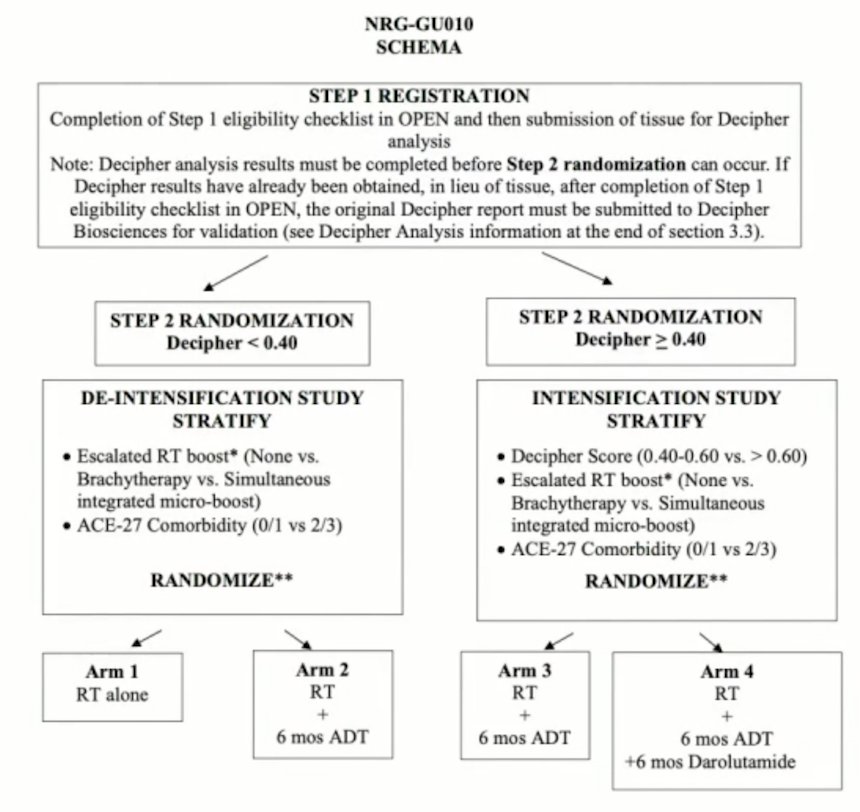
NRG GU-010 will require negative bone imaging (M0) within 120 days prior to registration, but note that Tc-99m bone scan or NaF PET are also allowed. While negative fluciclovine, choline, or PSMA PET may be counted as acceptable substitute for bone imaging, any suspicious findings must be confirmed and correlated with conventional imaging (Tc-99m bone scan, NaF PET, CT, X-ray, or MRI)) to determine eligibility based on the latter modalities. Additionally and similarly, while a negative fluciclovine, choline, or PSMA PET may be counted as acceptable substitute for pelvic imaging, any suspicious findings must be confirmed by conventional imaging (CT, MRI, or biopsy). If the findings do not meet pathological criteria based on the latter modalities (ie node <=10 mm in short axis, negative biopsy), the patient will still be eligible. The trial schema for NRG GU-010 is as follows:
For definitive patients, Dr. Ballas will:
- Order a PSMA PET/CT on high risk and very high risk patients, as well as some unfavorable intermediate risk patients
- Not just use PSMA PET/CT to drive the decision to treat the lymph nodes
- Treat the whole pelvis and boost PSMA positive lymph nodes (55.2 Gy in 23 fractions)
For post-op patients, Dr. Ballas will:
- Order a PSMA PET/CT in post-op patients with a PSA of >= 0.3 ng/mL
- Not just use PSMA PET/CT to drive the decision to treat
- Treat the whole pelvis and boost PSMA positive lymph nodes (55.2 Gy in 23 fractions)
In patients who have had prior prostate and or prostate bed radiotherapy and a recurrence is discovered in the pelvic lymph nodes, Dr. Ballas matches the radiotherapy fields and treats the entire pelvic lymph nodes with a boost to PSMA avid lymph nodes.
With regards to elective nodal radiotherapy versus stereotactic body radiotherapy, among <=5 lymph nodes, elective nodal radiotherapy is associated with fewer nodal recurrences compared to stereotactic body radiotherapy. Additionally, stereotactic body radiotherapy is associated with a 3-year MFS rate of 68% compared to 77% for elective nodal radiotherapy (p = 0.01). However, this comes at an increased risk of grade 2 late toxicity for elective nodal radiotherapy.
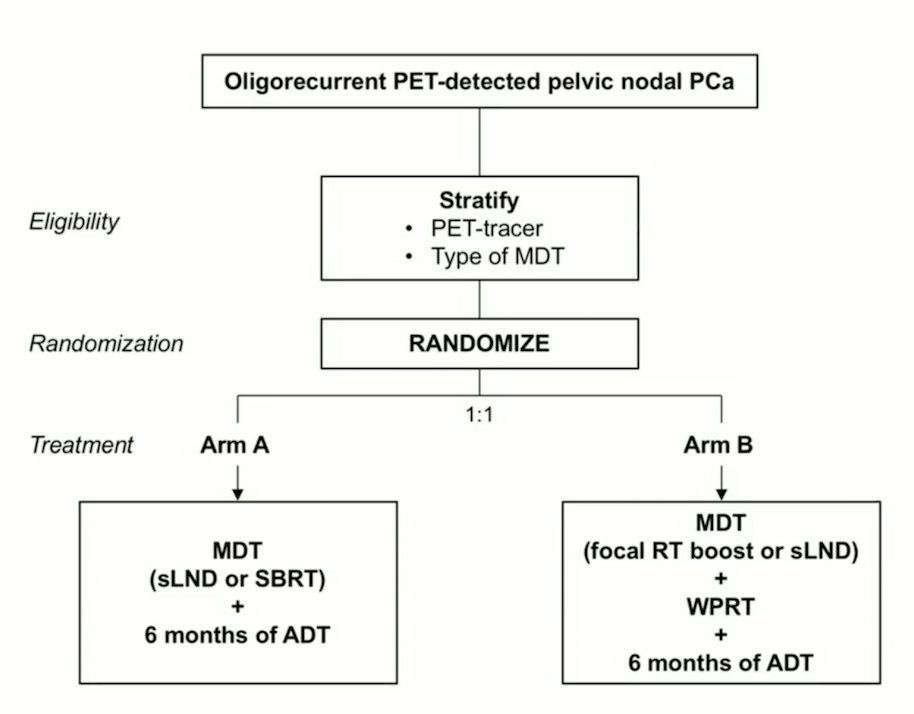
Peace V- STORM will randomize men with oligorecurrent PET-detected pelvic nodal prostate cancer (<= 5 nodes) 1:1 to metastasis directed therapy (salvage lymph node dissection or stereotactic body radiotherapy) + 6 months of ADT versus metastasis directed therapy (salvage lymph node dissection or focal radiotherapy boost) + whole pelvic radiotherapy (45 Gy in 25 fractions) + 6 months of ADT. The trial schema for Peace V- STORM is as follows:
Dr. Ballas notes that the surgeons have proven feasibility of salvage lymph node dissection, however the 5-year biochemical recurrence free survival ranges from only 6%-31%. Additionally, clinical recurrence-free and biochemical recurrence-free survival at 10 years are 31% and 11%, respectively. Ultimately, only 37% have a PSA response after salvage lymph node dissection.
Dr. Ballas concluded her presentation with two case presentations. The first case was a 69 year old male who had a TURP demonstrating Gleason grade 3 prostate cancer and a PSA of 91.1 ng/mL. A subsequent PSMA PET/CT scan showed a large intense active area involving most of the enlarged prostate gland consistent with prostate cancer. Additionally, there was intense metabolically active enlarged periprostatic lymph nodes, as well as intense metabolically active enlarge bilateral pelvic sidewall lymph nodes consistent with lymph node metastases:

This patient was then treated with 70 Gy in 28 fractions to the prostate, with 50.4 Gy to the pelvic lymph nodes with simultaneous integrate boost to 61.6 Gy (2.5 to the gland and 1.8 to the lymph nodes) with ADT.
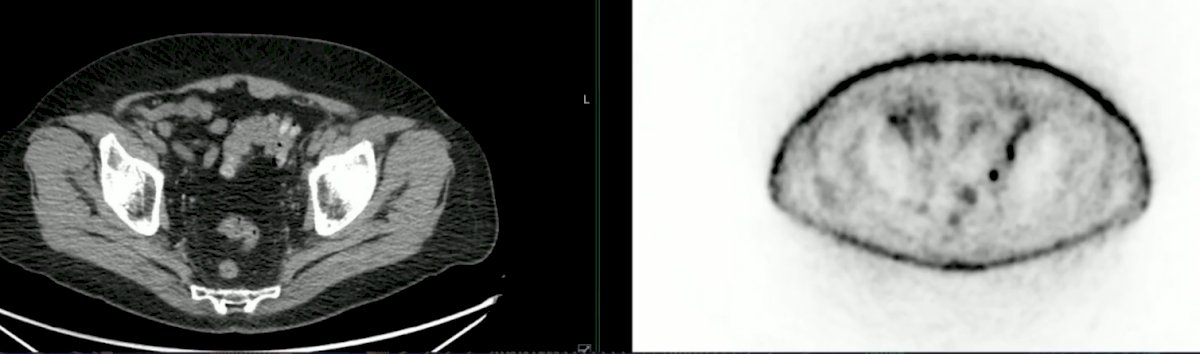
The second case was a 76 year old male with an elevated PSA (0.30 ng/mL) following HIFU for low-volume Gleason grade 4 prostate cancer. A PSMA PET/CT showed asymmetric moderate tracer accumulation left of midline at the apex of prostate suspicious for local recurrence. Additionally, there were several left-sided mildly tracer avid nodes in the pelvis suspicious for malignant disease:
This patient was subsequently treated with 60 Gy in 20 fractions to the prostate, and 44 Gy in 20 fractions to the pelvic lymph nodes with a simultaneous integrated boost to 55 Gy in 20 fractions.
Dr. Ballas concluded her presentation discussing treatment of local regional disease in the era of PSMA PET with the following concluding points:
- We have tools that helps identify nodal and metastatic diseases
- Using PSMA to deliver radiotherapy can help outcomes
- PSMA alone should not drive therapy decisions
Presented by: Leslie K. Ballas, MD, Cedars Sinai Medical Center, Los Angeles, CA
References:
- Hofman MS, Lawrentschuk N, Francis, RJ, et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): A prospective, randomized, multicentre study. Lancet 2020 Apr 11;395(10231):1208-1216.
- Jani AB, Schreibmann E, Goyal S, et al. 18F-fluciclovine-PET/CT imaging versus conventional imaging alone to guide postprostatectomy salvage radiotherapy for prostate cancer (EMPIRE-1): A single centre, open-label, phase 2/3 randomized controlled trial. Lancet. 2021 May 22;397(10288):1895-1904.


