(UroToday.com) The 2022 ASTRO annual meeting featured a prostate cancer session, including a presentation by Dr. Ashesh Jani discussing interim volumetric and toxicity analyses of a randomized trial of 18F-fluciclovine vs. 68Ga-PSMA PET/CT guided post-prostatectomy radiotherapy. Post-prostatectomy radiotherapy with 18F-fluciclovine (fluciclovine) has demonstrated improved event-free survival compared to conventional imaging (CT and/or MRI) based planning.1 Recently, 68Ga-PSMA (PSMA) has been explored as an alternative radiotracer. Dr. Jani and colleagues hypothesized that both fluciclovine and PSMA would result in significant changes in radiotherapy volumes and similar acute toxicity.
This was a prospective, randomized control trial in post-prostatectomy patients with biochemical relapse comparing fluciclovine (Arm 1) and PSMA (Arm 2) incorporation. Target volume changes before (pre) and after (post) PET incorporation were assessed including prostate bed with (CTVPLV/ PTVPLV) or without (CTVPB/PTVPB) pelvic nodal treatment. PTVPB received 66.6Gy +/- boost up to 76 Gy and PTVPLV received 45Gy +/- boost up to 56 Gy. Dr. Jani and colleagues evaluated organ-at-risk V40Gy and V65Gy pre and post for: bladder (-CTV), rectum, penile bulb, and V45Gy for small bowel. Acute provider reported (<30 day) toxicity was assessed using CTCAE v5.0. Fisher's exact test & Wilcoxon signed-rank test were used. This analysis includes 100 of 140 intended patients.
Of 100 patients accrued 23 patients were excluded for extra-pelvic uptake, consent withdrawal, pending radiotherapy or were not yet randomized (11 Arm A and 12 Arm B) leaving 77 patients for analysis (39 Arm 1 and 38 Arm 2). Arm 1 patients had significantly increased mean CTVPB (115.99 cc vs 116.72 cc; pre vs post, respectively; p < 0.01); mean PTVPB (282.26 cc vs 286.02 cc, pre vs post, respectively; p < 0.01), mean CTVPLV (474.58 cc vs 477.72 cc; pre vs post, respectively; p < 0.01) and mean PTVPLV (1063.65 cc vs 1073.17 cc, pre vs post, respectively; p = 0.01) volumes. Arm 2 patients had increased mean CTVPB (142.53 vs 146.32 cc; pre vs post, respectively; p < 0.01), however, there was no significant difference in mean PTVPB (p = 0.18); mean CTVPLV (p = 0.63); or mean PTVPLV (p = 0.22) volumes:
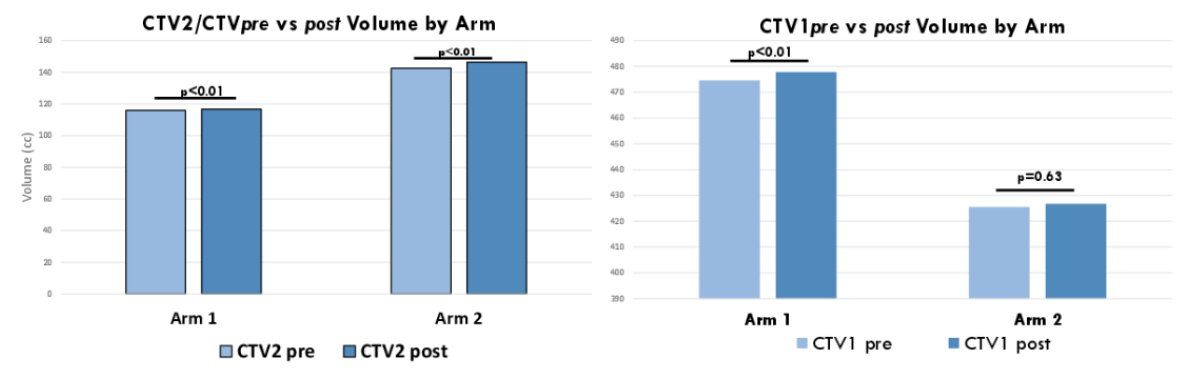
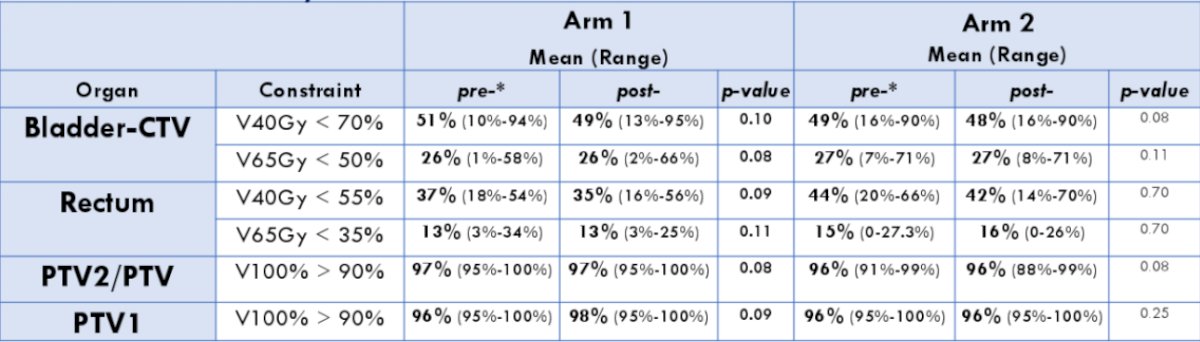
Rectal V65Gy (p < 0.01) and penile bulb V40Gy and V65Gy (p < 0.01) were increased in Arm 1 post vs pre, but were within trial constraints. No other organ-at-risk constraints were significantly different pre vs post in either Arm:
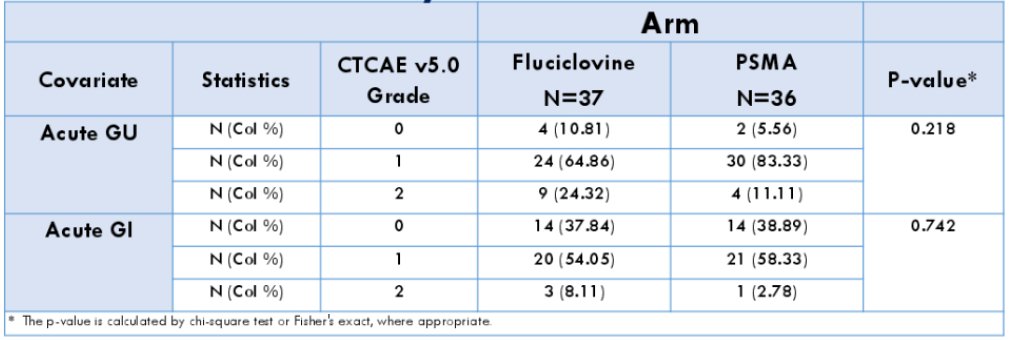
Grade 2 genitourinary (24.32% vs 11.11%, Arm 1 vs Arm 2, respectively) and gastrointestinal (8.11% v 2.78%, Arm 1 vs Arm 2, respectively) toxicity was moderate; overall toxicity was not significantly different between Arms (p = 0.22 vs 0.74, genitourinary and gastrointestinal, respectively) with no grade 3+ events reported:
Dr. Jani concluded his presentation discussing interim volumetric and toxicity analyses of a randomized trial of 18F-fluciclovine vs. 68Ga-PSMA PET/CT guided post-prostatectomy radiotherapy with the following concluding messages:
- Both fluciclovine and PSMA guided radiotherapy planning impacted bed target volume
- Due potentially to prostate bed uptake differences (in an earlier report of the first 65 patients PET uptake was 84.4% vs 39.4% for prostate bed in Arm 1 vs 2, respectively), Arm 1 patients had significantly increased PTVPB and PTVPLV volumes post-PET
- There was no significant difference in acute toxicity between arms
- Further analyses of biochemical control and patient reported outcomes are forthcoming
Presented by: Ashesh Jani, MD, MSEE, FASTRO, Department of Radiation Oncology, Emory University, Atlanta, GA
Co-Authors: V. R. Dhere1, D. M. Schuster2, S. Goyal3, E. Schreibmann1, B. Hershatter4, S. A. Patel1, J. W. Shelton1, S. Hanasoge1, P. R. Patel1, N. Sebastian5, O. A. Adediran6, and I. O. Lawal6; 1Department of Radiation Oncology, Winship Cancer Institute of Emory University, Atlanta, GA, 2Department of Radiology and Imaging Sciences, Emory University, Atlanta, GA, 3Department of Biostatistics and Bioinformatics Shared Resource, Winship Cancer Institute, Atlanta, GA, 4Winship Cancer Institute of Emory University, Atlanta, GA, 5Department of Radiation Oncology, The Ohio State University Wexner Medical Center, Columbus, OH, 6Emory University, Atlanta, GA
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 American Society of Radiation Oncology (ASTRO) Annual Hybrid Meeting, San Antonio, TX, Sat, Oct 22 – Wed, Oct 26, 2022.
References:
- Jani AB, Schreibmann E, Goyal S, et al. 18F-fluciclovine-PET/CT imaging versus conventional imaging alone to guide postprostatectomy salvage radiotherapy for prostate cancer (EMPIRE-1): A single centre, open-label, phase 2/3 randomized controlled trial. Lancet. 2021 May 22;397(10288):1895-1904.


