(UroToday.com) The 2022 American Society for Radiation Oncology (ASTRO) Annual Meeting was host to a late-breaking abstract session, during which Dr. Igor Latorzeff presented results of the GETUG-AFU 22 trial. Dr. Latorzeff began his presentation by highlighting that PSA persistence post-radical prostatectomy is distinct from biochemical recurrence. PSA persistence occurs in 5 to 20% of patients undergoing a radical prostatectomy, with these patients having a post-operative PSA >0.1 ng/ml 6 to 8 weeks after surgery. Approximately 75% of patients with PSA persistence ultimately develop biochemical recurrence. In multivariable models, PSA persistence has been demonstrated to be an independent predictor of metastasis and cancer-specific mortality with significant effect sizes (hazard ratios > 3).
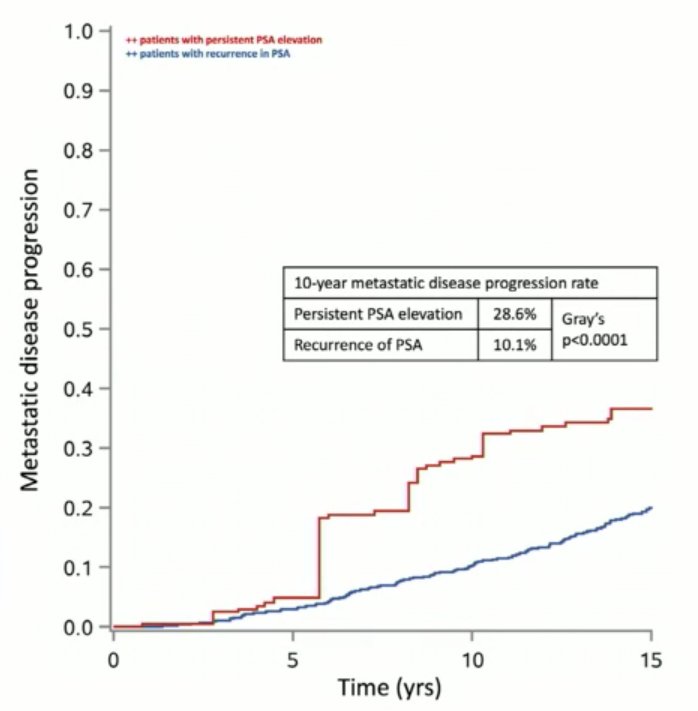
Results from the RTOG-9601 trial demonstrated that men with PSA persistence (nadir PSA >0.4 ng/ml) had significantly worse outcomes compared to men with biochemical recurrence (nadir PSA <= 0.4 ng/ml). In this trial, all patients received salvage radiotherapy +/- two years of bicalutamide. After a median follow-up of 12 years, the 10-year local recurrence (3.2% versus 1.4%, p=0.001) and metastatic rates (28.6% versus 10.1%, p<0.001) were worse in the PSA persistence group.1 Dr. Latorzeff highlighted that, as of yet, there have been no RCTs assessing the role of salvage radiotherapy in patients with PSA persistence.
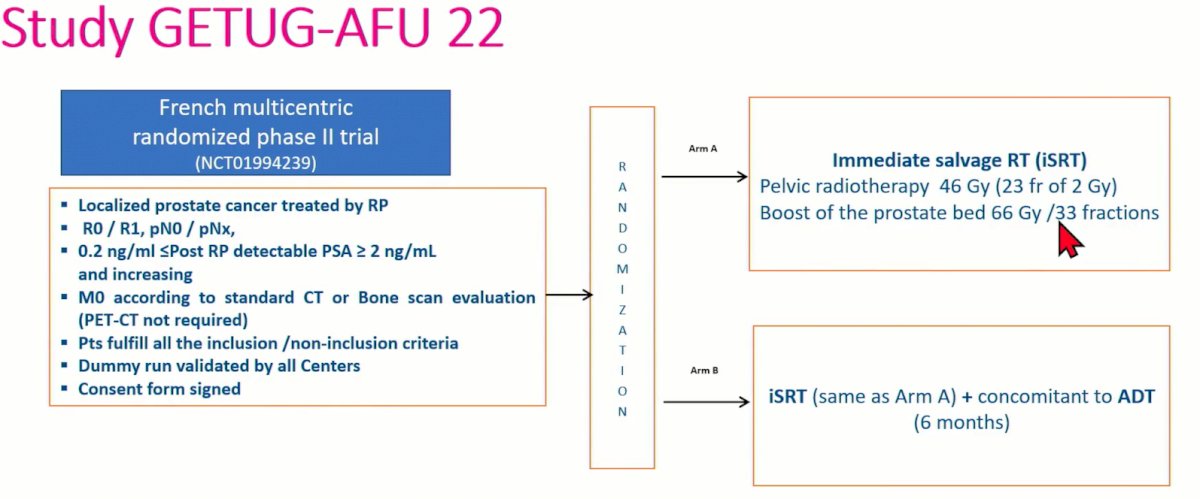
Dr. Latorzeff next presented the study design of the GETUG-AFU 22 trial. In brief, this was a French, multicenter, randomized, phase II trial (NCT10994239) that included patients with localized prostate cancer treated with a radical prostatectomy. Patients were included irrespective of margin status and had pN0/x disease. Patients had PSA persistence post-op with a PSA level between 0.2 and 2 ng/ml and increasing post-operatively. Staging work-up with conventional techniques (CT/bone scan) were negative, as PSMA PET was not available at time of study design. Patients were randomized in a 1:1 fashion to either immediate salvage radiation therapy (pelvic 46 Gy + prostate bed boost 66 Gy/33 fractions) with or without 6 months of ADT (degarelix). The primary endpoint was event-free survival, defined as biochemical progression, clinical progression, use of ADT, or any cause death. Secondary endpoints included 5-year event-free survival and metastasis-free survival, 5- and 10-year overall survival, acute and late toxicities, and the correlation between testosteronemia and PSA kinetics.
The study was designed with the following assumptions:
- Accrual period of 2 years
- An exponential event-free survival in the two arms
- A median event fee survival of 36 months in the least effective arm
- A median event-free survival of 45 months in the most effective arm
- An analysis planned 5 years after the first inclusion
- A 5% rate of patient dropout
Based on the sample size calculations, 122 patients were required to select the most effective arm with a power of at least 80% for a 20% reduction in the instantaneous risk ration (HR: 0.80).
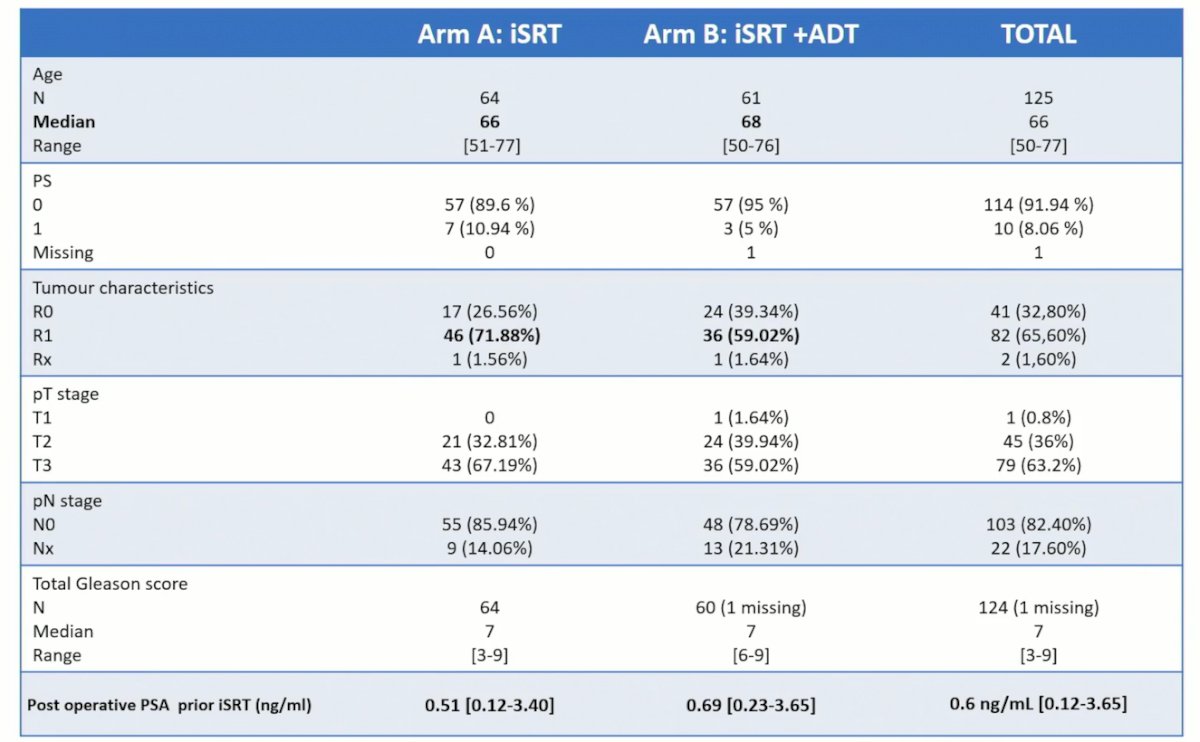
Between Dec 2012 and September 2015, 125 patients were included (64 in the XRT only arm and 61 in the XRT+ADT arm). The median follow-up was 74.94 months (95% CI: 74.1 – 76.6). With respect to treatment regimens, 3D-RT was recommended in 18% and IMRT in 82%. For PTV1 (pelvis + prostate bed), 46 Gy in 23 fractions was given. Boost target volumes (PTV2) to the prostatectomy bed was given as 66 Gy in 33 fractions. ADT was given in the form of degarelix (GnRH antagonist) at an initial dose of 240 mg in 2 injections of 120 mg, followed by a maintenance dose of 80 mg monthly.
Overall, patient baseline characteristics were well-balanced between the two arms. Median patient age was 66 years. Notably, post-operative PSA was slightly higher in the ADT arm (0.69 versus 0.51 ng/ml), but positive margin status was comparatively higher in the no ADT arm (72% versus 59%).
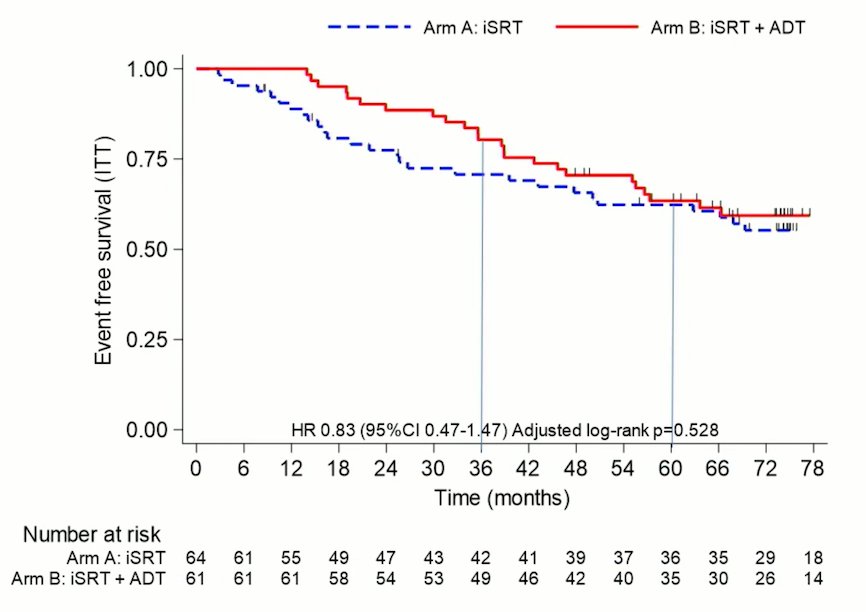
This study did not meet its primary endpoint of event-free survival. Median EFS was not reach in both arms. 5-year EFS in the XRT +ADT arm was 63.4% (95% CI: 49.9 – 74.2%) versus 62.3% (95% CI: 48.9 – 73.2%) in the XRT alone arm (HR: 0.83; 95% CI: 0.47 – 1.47, p=0.53). With 53 events, the probability that combination salvage XRT + ADT is actually better than salvage XRT alone in the population is 79%. As such, salvage XRT + ADT may be used as a reference arm for a phase III study
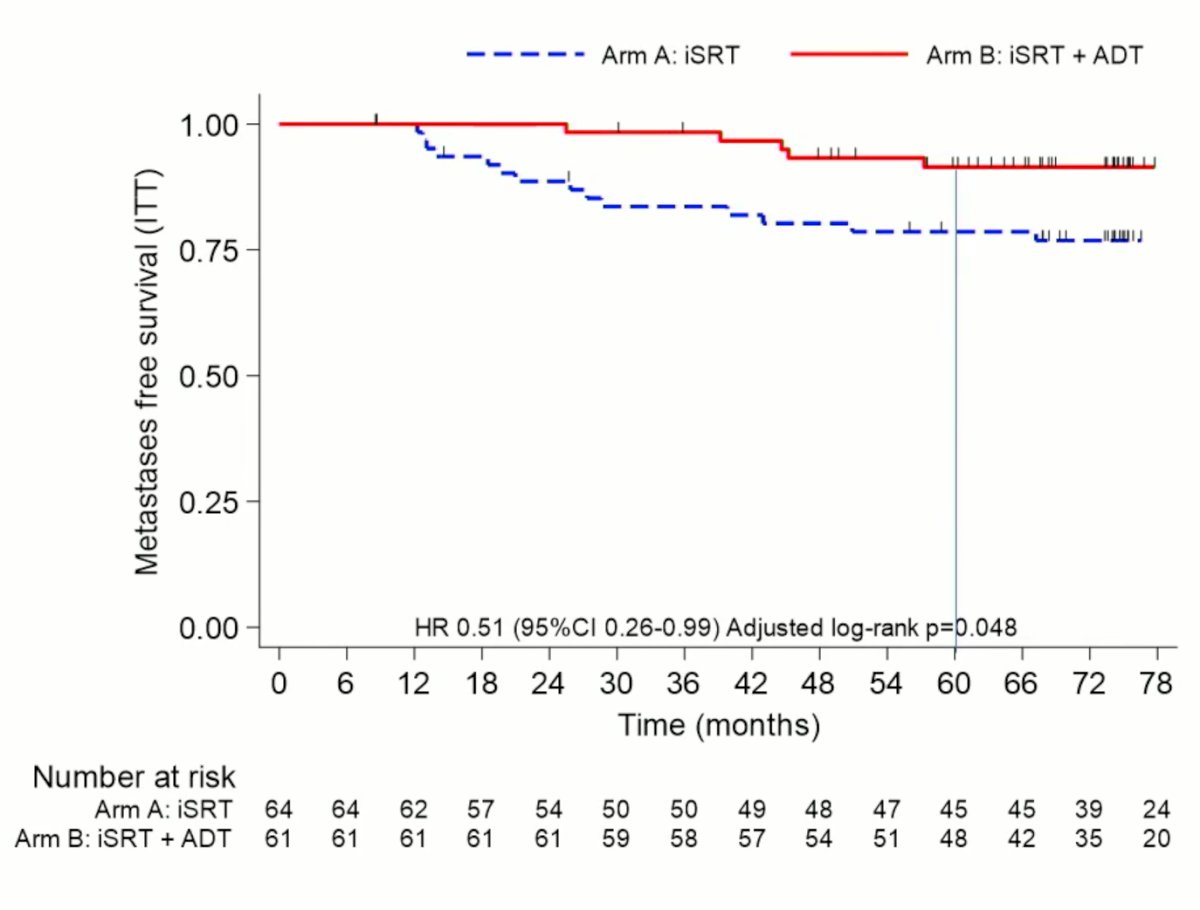
With regards to the key secondary endpoint of metastasis-free survival, addition of ADT was associated with significant improvements (HR: 0.51, 95% CI: 0.26 – 0.99, p=0.048). The metastasis-free survival rates at 12, 36 and 60 months were 100% in both arms, 98.4% versus 83.6%, and 91.4% versus 78.6% (both in favor of ADT addition). On multivariate analysis, predictors of metastasis included addition of ADT (HR: 0.39, 95% CI: 0.16 – 0.93, p=0.028) and PSA prior to salvage XRT (HR for >= 0.6 versus <0.6: 2.82, 95% CI: 1.14 – 6.95, p=0.019).
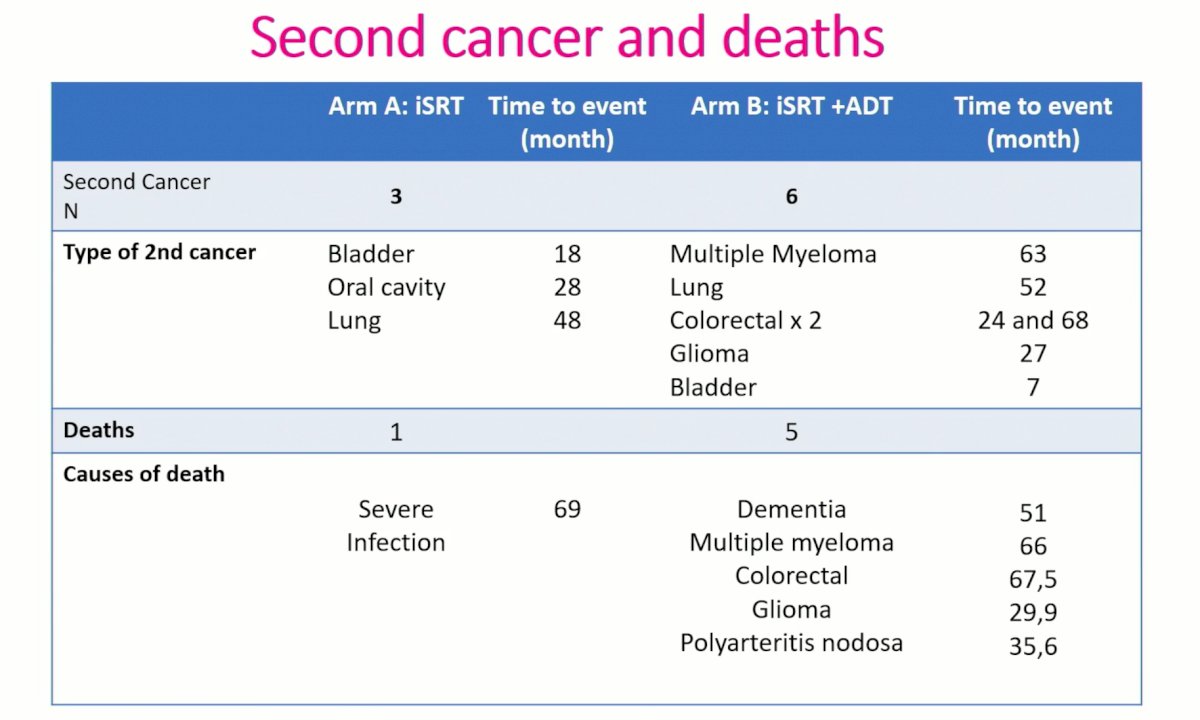
Dr. Latorzeff noted that late toxicity and QoL outcomes had previously been presented at ASTRO 2019, with later toxicity assessed by physicians noted to be low and no difference was noted between the two arms at 2 years for GU/GI or maximal toxicity. QoL assessed with questionnaires showed an impact of adverse effect for ADT within one year, but no difference with prolonged follow up. With respect to second cancer and deaths outcomes. Notably, patients in the combination arm had 6 second primary cancers versus 3 in the XRT only arm. 5 patients died in the combination arm as opposed to 1 in the XRT only arm.
Dr. Latorzeff concluded his presentation as follows:
- GETUG-AFU 22 is the first prospective randomized phase II trial comparing immediate salvage radiation therapy with or without ADT (degarelix) in patients with PSA persistence post-radical prostatectomy.
- For the primary endpoint of event-free survival, median survival durations were not reached. With 53 events, the probability that combination salvage XRT + ADT is actually better than salvage XRT alone in the population is 79%. As such, salvage XRT + ADT may be used as a reference arm for a phase III study
- Secondary endpoints (MFS), as a predictor marker for survival, favored the addition of short-term ADT to salvage radiation therapy in this population.
- Current ongoing trials in this disease space include the phase II trials STEELE, Formula-509 and BALANCE, along with the phase II-III trial NRG-GU002.
Presented by: Igor Latorzeff, MD, Department of Radiation Oncology, Clinique Pasteur Toulouse, Toulouse, France
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2022 American Society of Radiation Oncology (ASTRO) Annual Hybrid Meeting, San Antonio, TX, Sat, Oct 22 – Wed, Oct 26, 2022.
References:- Shipley WU, et al. Radiation with or without Antiandrogen Therapy in Recurrent Prostate Cancer. N Engl J Med. 2017;376:417-428.


