(UroToday.com) The 2022 American Society for Radiation Oncology (ASTRO) Annual Meeting held in San Antonio, TX between October 23rd and 26th, 2022 was host to a State of the Art session for the selection and treatment of localized prostate cancer. Dr. Brett Morris presented results of a phase II trial evaluating the toxicity and patient reported quality of life outcomes following prostate SBRT with focal boost to MRI identified prostate cancer lesions.
Dr. Morris began his presentation by noting that focal boost radiation to MRI-visible prostate cancer lesions improves disease control with acceptable toxicity. Results from the FLAME trial, which utilized a focal boost with standard fractionation, improved 5-year biochemical disease-free survival from 85% to 92% with low rates of late toxicity.1 More recently, use of SBRT in the HypoFlame trial (35 Gy in 5 fractions once weekly with a focal boost up to 50 Gy in 5 fractions) demonstrated no grade 3 or worse acute GU or GI toxicity, with acute grade 2 GU toxicity of 34% and acute grade 2 GI toxicity of 5%.2
The authors thus sought to evaluate whether heterogenous radiation therapy to the prostate with focal boost would provide a similar favorable toxicity profile without compromising oncologic outcomes. The authors treated the entire gland to 40 Gy in 5 fractions (green region below). Areas at high risk of toxicity (urethra, bladder, and rectum) were treated with dose de-escalation to 36.25 Gy in 5 fractions. MRI detected PIRADS 4 and 5 lesions were treated with a focal boost to 45 Gy in 5 fractions.

Eligible patients were those with Gleason score of 7 or less, PSA <20 ng/ml, ≤cT2b disease, and AUA score <18. Use of ADT was optional. Patients in Arm A (preferred/MRI arm) received heterogenous SBRT with 40 Gy in 5 fractions to the prostate, 36.25 Gy in 5 fractions within 2 mm of the urethra, bladder, and rectum with focal boost to 45 Gy to MRI-identified PIRADS 4-5 lesions. Those in Arm B (No MRI arm) received 37.5 Gy in 5 fractions to the prostate, and 36.225 Gy in 5 fractions within 2 mm of the areas at risk. Toxicity and patient reported quality of life outcomes were collected at 6-month intervals for 5 years.
The study included 114 patients, with 100 (87.7%) in Arm A and 14 (12.3%) in arm B. Majority (73.7%) were cT1c, and 87% had Grade Group 2 disease. Median pre-treatment PSA was 9.0 ng/ml. 33% of patients received ADT with a median duration of 4 months.
With respect to oncologic outcomes, the biochemical disease-free survival at a median follow-up of 36 months was 97.4%, with 3 biochemical recurrene events encountered.

None of the patients experienced grade 3+ GU or GI toxicity. Acute grade 2 GU and GI toxicities were noted in 38% and 3.5% of patients, respectively.

Similarly, no late grade 3 GU or GI toxicities were noted. The cumulative incidence of grade 2 late GU and GI toxicities were 9.3% and 2.8%, respectively.
With regards to patient reported outcomes, there were no significant changes in GU quality of life as determined by the EPIC-26 Urinary Incontinence Score or EPIC-26 Urinary Irritative Score.

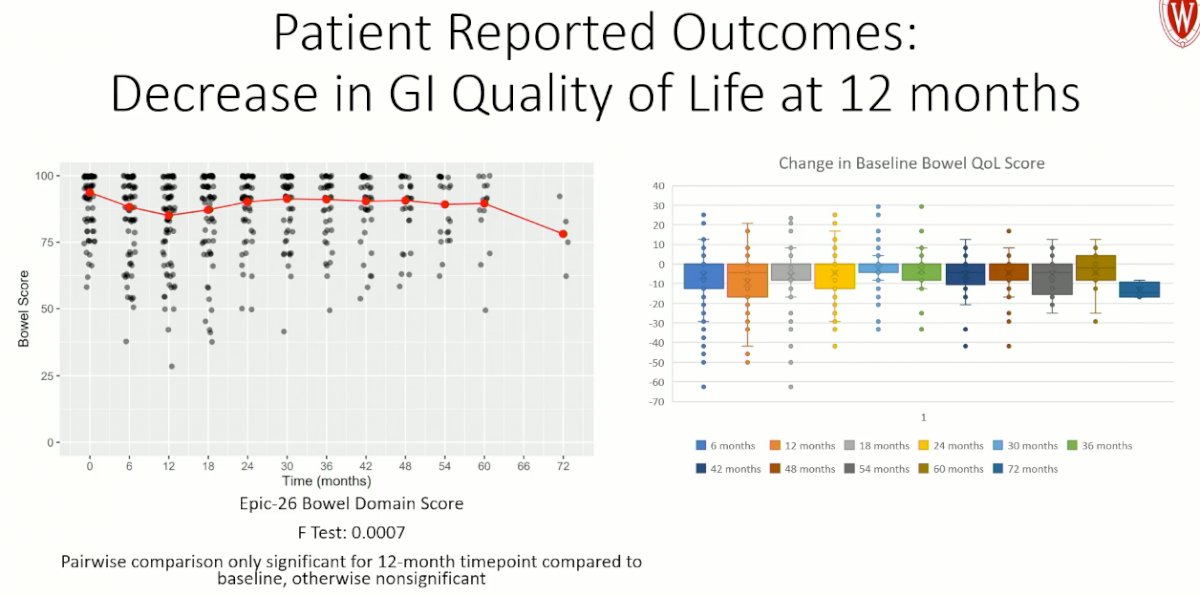
There was a significant worsening in GI quality of life at 12 months, compared to baseline, as determined by the EPIC-26 bowel domain score.
There were no changes in patient reported sexual health or hormonal quality of life, as determined by the EPIC-26 Sexual Health Domain and the EPIC-26 Hormonal Domain scores.

Dr. Morris concluded as follows:
- Prostate SBRT using a focal boost to MRI areas of disease with dose de-escalation to areas adjacent OARs is technically feasible
- Acute and late toxicity rates are similar to previously reported SBRT studies
- Patient reported quality of life measures shows no long-term significant changes from baseline
- Disease control is excellent at a median follow up of 36 months
Presented by: Brett A. Morris, MD, PhD, Radiation Oncology Resident Physician, Department of Human Oncology, University of Wisconsin School of Medicine and Public Health, Madison, WI
Written by: Rashid Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2022 American Society of Radiation Oncology (ASTRO) Annual Hybrid Meeting, San Antonio, TX, Sat, Oct 22 – Wed, Oct 26, 2022.
References:
- Kerkmeijer LGW, et al. Focal Boost to the Intraprostatic Tumor in External Beam Radiotherapy for Patients With Localized Prostate Cancer: Results From the FLAME Randomized Phase III Trial. J Clin Oncol. 2021;39(7):787-96.
- Draulans C, et al . Primary endpoint analysis of the multicentre phase II hypo-FLAME trial for intermediate and high risk prostate cancer. Radiothera Oncol. 2020;147:92-8.


