(UroToday.com) The 2023 American Society for Radiation Oncology (ASTRO) 65th Annual Meeting held in San Diego, CA between October 1st and 4th, 2023 was host to a session on radiotherapy for kidney cancer and in the post-prostatectomy setting. Dr. Anand Swaminath presented the final results of RADSTER, a prospective randomized pilot trial of stereotactic body radiotherapy (SBRT) versus radiofrequency ablation for the management of small renal masses.
Dr. Swaminath began by highlighting that there is an increased incidence of small renal masses, secondary to the increased utilization of cross-sectional abdominal imaging. Currently, surgery remains the standard of care for localized, non-metastatic RCC with nephron-sparing approaches most commonly used for patients with small (4 cm or less) renal masses via a partial nephrectomy, with excellent long-term results. However, some patients may be at higher risk for surgery-related complications, including those with chronic renal dysfunction, elderly age, medical comorbidities, and poor performance status. Active surveillance remains an option for these patients, but as tumors grow larger (>4 cm), particularly in the setting of fast growth rates (>1 cm/year), the need to evaluate other oncologic therapies becomes more pressing.
Currently, there are two potential non-surgical treatment options for this patient population:
- Radiofrequency ablation (RFA): A form of thermal ablation using a radiofrequency probe directed to small renal masses.
- Demonstrated good oncologic outcomes in retrospective series (>90% disease-free survival for masses 3 cm or less)
- However, has some limitations – location of tumors that are adjacent to the renal hilum and bowel being technically challenging and some limitations with regards to larger tumor sizes that may require a repeat ablation
- SBRT: Employs high radiotherapy doses per fraction with highly conformal radiation in few fractions (5 or less)
- Pooled retrospective data from IROCK: Similar control rates with RFA (>90%, regardless of tumor size) with minimal toxicity and potentially better oncologic outcomes with single versus multifraction regimens.1
- There are some limitations: Concerns regarding ‘radio-resistance’ persist, concerns about persistent enhancement following treatment, and worsening of renal dysfunction/toxicity
To date, there are no published direct comparisons of SBRT with other treatment modalities. This provided the impetus for RADSTER, which is a prospective, pilot randomized trial comparing RFA to SBRT for patients with 4 cm or smaller renal masses. This trial planned to enroll 24 patients meeting the following inclusion criteria:
- Biopsy proven RCC
- Tumors 4 cm or less
- Primary kidney lesion amenable to SBRT or RFA
- Patients willing to have a biopsy at 1 year
Patients were planned for 1:1 randomization to either SBRT (25 Gy in 1 fraction) or RFA in a single session. The feasibility study endpoints included:
- Safety
- Recruitment rates
- Consent rates
- Completion rates (based on imaging and 1-year biopsy compliance)
The clinical endpoints included:
- Disease-free survival (based on imaging and 1-year biopsy)
- Quality of life
- Renal toxicity
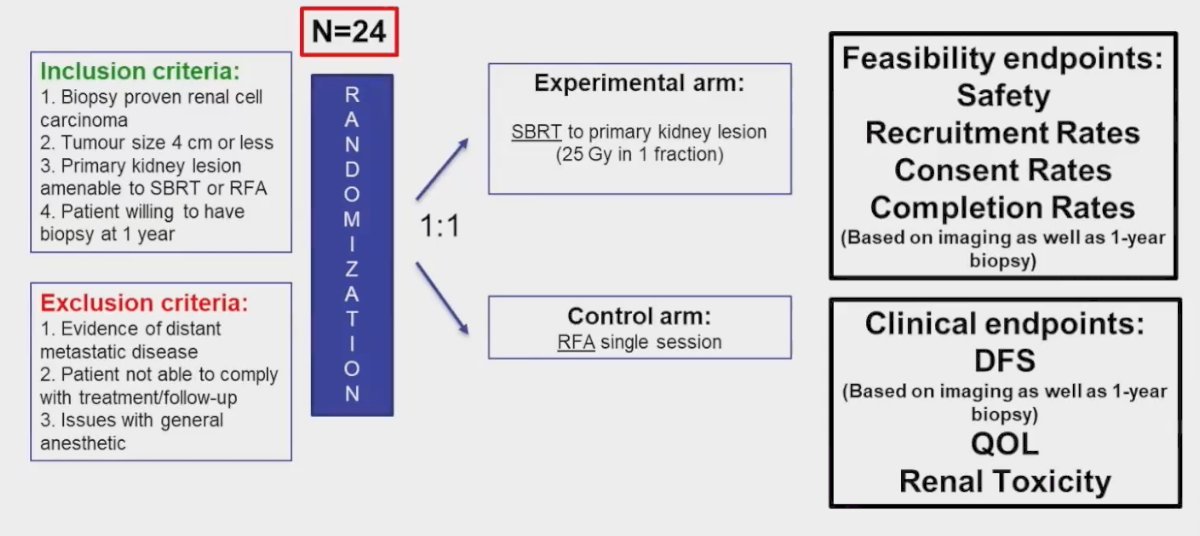
The technical details to the RFA and SBRT treatment approaches are summarized in the image below:
Between January 2020 and June 2021, 33 patients were screened, of whom 24 were enrolled. These patients were randomized in a 1:1 fashion to SBRT (n=12) or RFA (n=12). However, 3 patients randomized to RFA were subsequently treated with SBRT due to technical non-amenability to RFA at the time of the procedure. Furthermore, 1 patient randomized to SBRT was not amenable due to intolerance of immobilization/positioning due to frailty, and 2 patients in the RFA arm opted for surgery and active surveillance after being randomized. At the end, 14 patients received SBRT and 7 underwent RFA.
Baseline patient demographics are summarized below. The mean patient age was 67 – 70 years. The mean tumor size was 2.4 cm. Of note, the mean time from enrolment to treatment was approximately 3 months, although we note that this trial was conducted in patients at low risk of metastasis during the COVID-19 pandemic.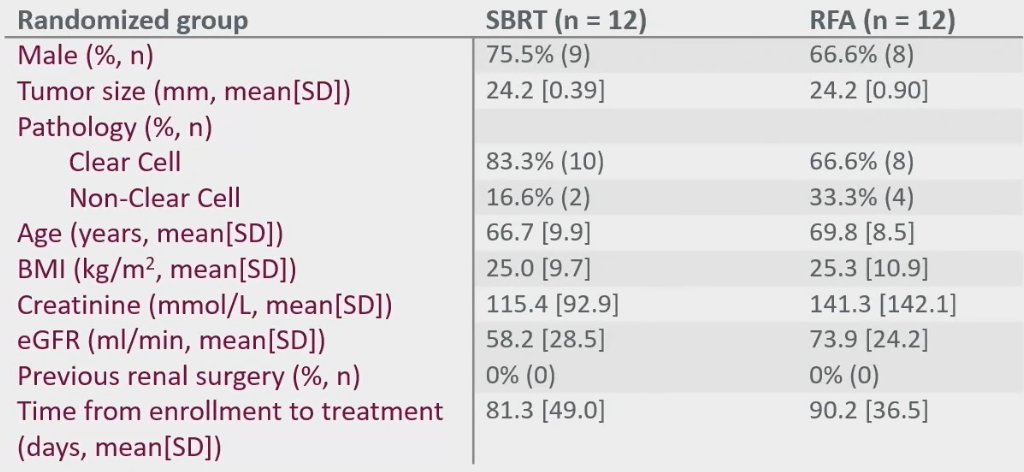
With regards to toxicity and adverse events, the authors observed no significant differences in toxicity outcomes:
- RFA: No clinically significant acute or late toxicity at 1 year with RFA
- SBRT: 1 patient with acute grade 2 chest wall pain. No late toxicity up to 1 year with SBRT.
With regards to renal function outcomes at 1 year, there was a similar mean decline in eGFR (-3.0 in RFA and -5.2 in SBRT, p=0.70). Of note, quality of life data is pending.
With regards to treatment efficacy outcomes, the pathologic complete response at the mandated 1-year biopsy demonstrated significantly worse outcomes in the SBRT group (33% versus 100% in RFA, p=0.01). Furthermore, radiologic response occurred less frequently in the SBRT group (23% versus 83.3%, p=0.04). There were no significant differences in the other assessed efficacy outcomes, including progression-free and overall survivals.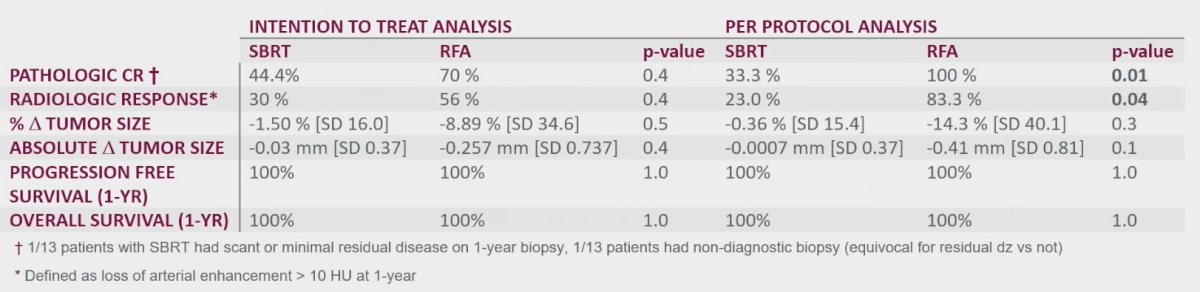
Dr. Swaminath concluded his presentation with the following:
- A trial of SBRT versus RFA for small renal RCC masses is feasible locally, and most patients will accept randomization if amenable to that treatment
- Virtually all patients who are treatable with RFA are also feasible to be treated with SBRT
- Even those who are not RFA eligible technically can receive SBRT safely
- Despite a pandemic, recruitment was steady and feasible
- There are plans underway to expand this trial to a multicenter RCT: RADSTER-2
- Non-inferiority trial of SBRT versus cryoablation or RFA
- This trial will incorporate novel imaging and biomarkers with pathologic secondary objectives
- Further final analysis of the current trial is underway, with assessment of long-term disease control, radiologic response, and quality of life outcomes
Presented by: Anand Swaminath, MD, Associate Professor, Department of Radiation Oncology, Juravinski Cancer Centre, Hamilton, ON
Written By: Rashid K. Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 American Society for Therapeutic Radiation Oncology (ASTRO) 65th Annual Meeting held in San Diego, CA between October 1st and 4th, 2023
References:

