(UroToday.com) The 2023 American Society for Radiation Oncology (ASTRO) 65th Annual Meeting held in San Diego, CA was host to a session on stereotactic radiotherapy for renal cell cancer. Dr. Rohann Correa provided a primer on primary kidney stereotactic ablative radiotherapy (SABR).
Dr. Correa began by highlighting that the incidence of renal cell carcinoma (RCC) is increasing in North American, Europe, and Asia, likely secondary to the increased utilization of axial imaging in clinical practice. This increase is most pronounced in those older than 70 years of age, who are at a greater risk of cancer-specific mortality (up to 3.8-fold).1 Furthermore, their advanced age, along with increased frailty and comorbidities may preclude surgical or percutaneous interventions.

Dr. Correa next addressed the ‘elephant in the room’ – Is RCC not radioresistant? RCC was historically considered radioresistant, but to standard fractionation radiotherapy administered at doses of 1.8 – 2.0 Gy. However, as apparent in the curve below, higher doses per fractionation (>6 Gy) produces logarithmic cytotoxicity.
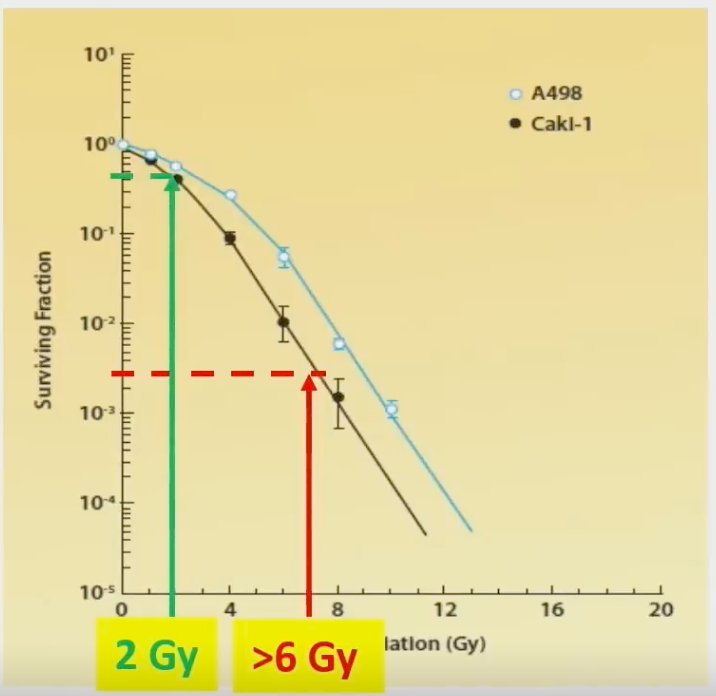
As such, SABR can overcome radioresistance by promoting tumor vascular collapse and anti-tumor immunity.
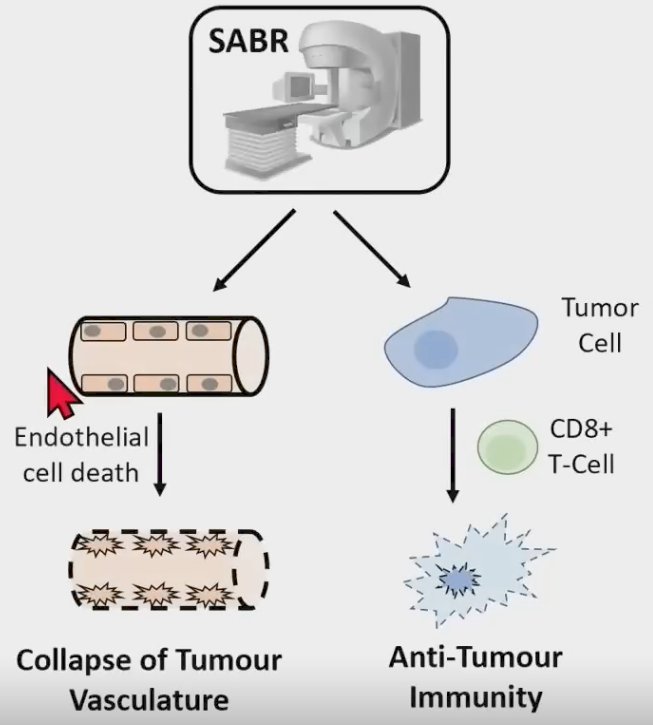
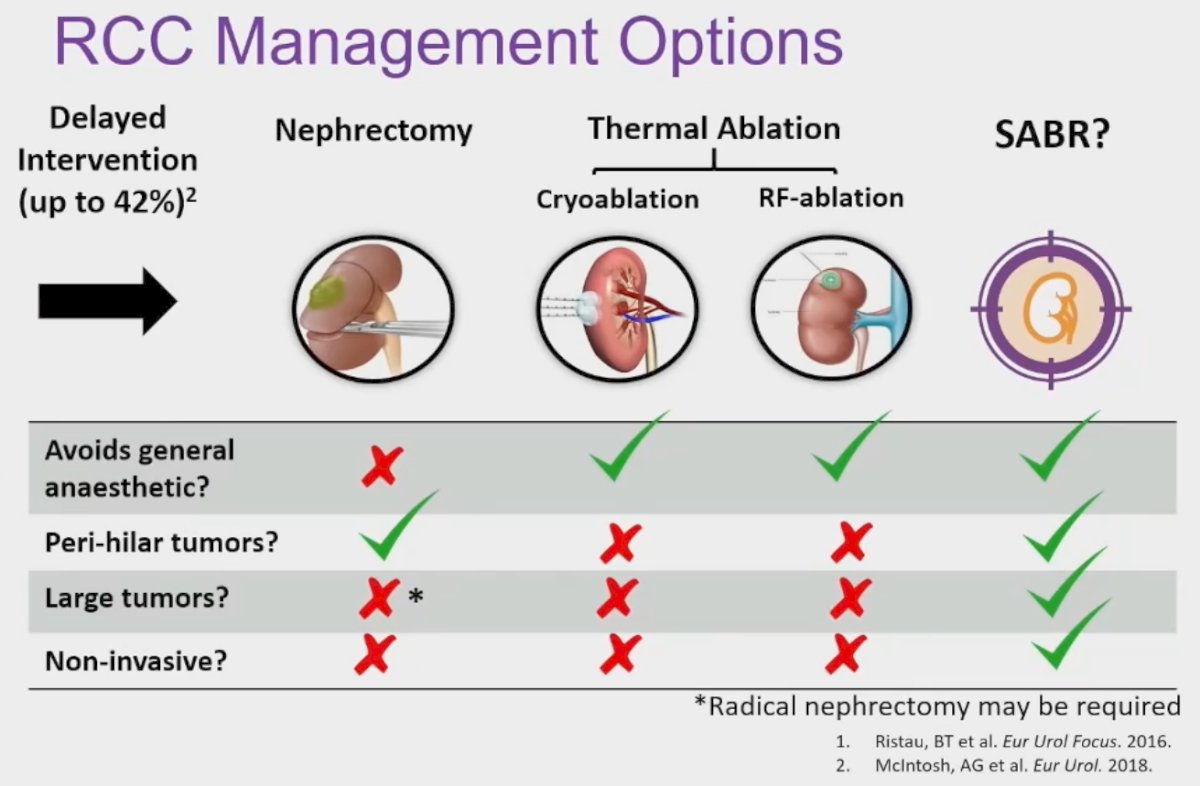
Currently, available management options for RCC patients have several important limitations. Nephrectomy requires general anesthesia and can be morbid in these elderly, frail patients. Conversely, thermal ablative procedures, such as radiofrequency ablation and cryoablation, can be technically challenging for larger, perihilar tumors with worsening efficacy outcomes with increasing sizes. SABR, which entails image-guided, precision targeted delivery of high radiotherapy doses in limited fractions, may be an attractive treatment option in this setting, given that this treatment modality avoids general anesthesia, is feasible for large, perihilar tumors, and is non-invasive.
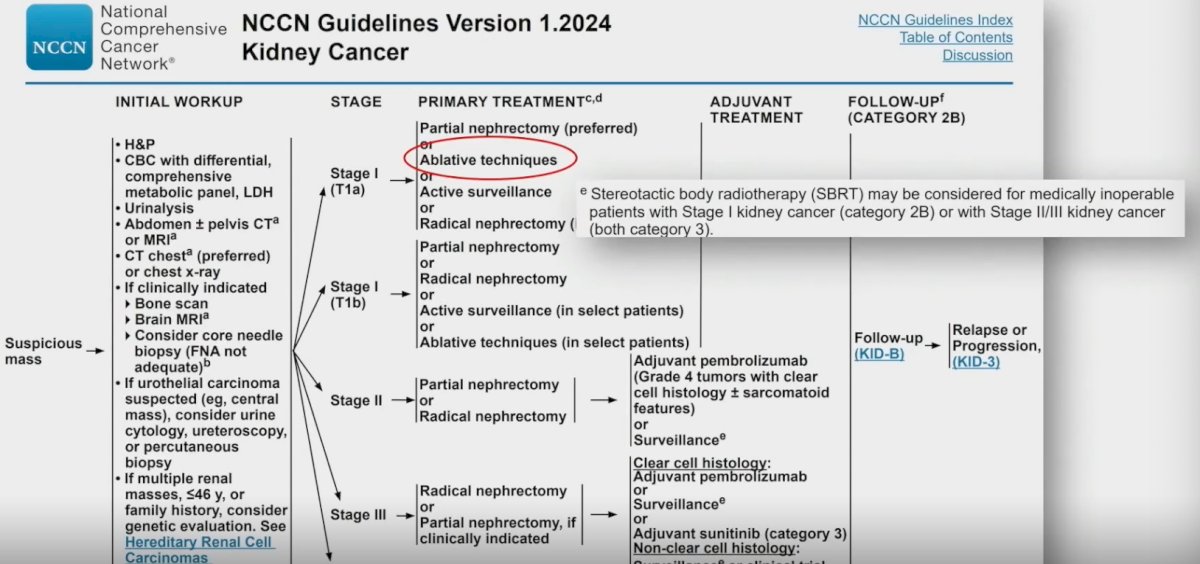
Current guidelines, including those of the National Comprehensive Cancer Network (NCCN), have acknowledged SABR as a potential treatment option for patients with stage cT1a disease, who are considered medically inoperable.
Dr. Correa next proceeded to provide a historical context for SABR in RCC patients. In 2003, we witnessed the first report of SABR for the targeting of primary RCC tumors, with the first report of SABR for extra-cranial oligometastases subsequently published in 2005. In 2006, the first prospective trial of SABR for the targeting of primary RCC tumors was published. These efforts culminated in the iROCK international consensus statement on SABR for primary RCC in 2016 and the subsequent publication of an individual patient data meta-analysis of SABR for primary RCC published in The Lancet Oncology in 2022.2
This study was an individual patient data meta-analysis, for which patients undergoing SABR for primary renal cell carcinoma across 12 institutions in five countries (Australia, Canada, Germany, Japan, and the USA) were eligible. Included patients had at least 2 years of follow-up, were aged 18 years or older, had any performance status, and had no previous local therapy. SABR was delivered as a single or multiple fractions of greater than 5 Gy. The primary study endpoint was investigator-assessed local failure per RECIST version 1.1. This analysis included 190 patients, followed for a median of 5.0 years. The median age at baseline was 73.6 years. The median tumor size was 4 cm. Notably, pathologic confirmation was performed in 83% of patients and 75% were deemed medically inoperable. The median total dose administered was 30 Gy either in single or multiple fractions, as summarized below:
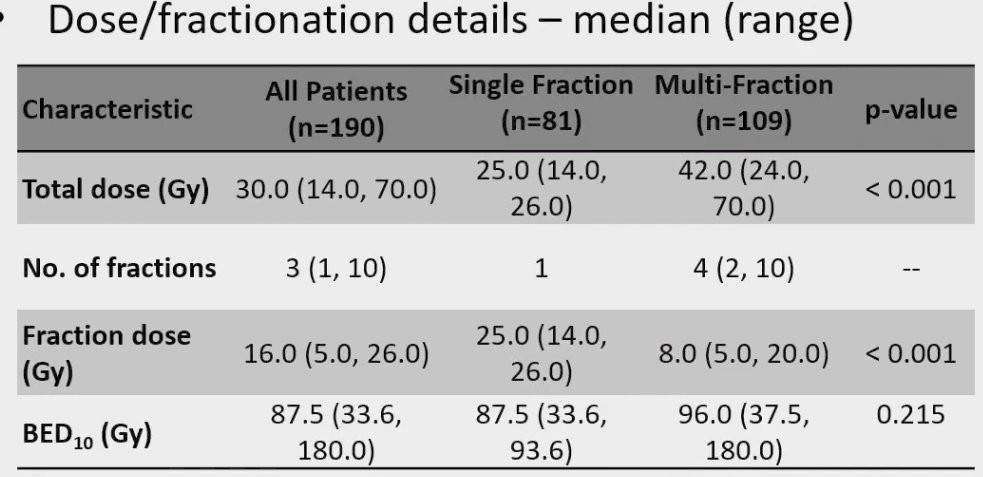
In this cohort, the 5-year cancer-specific survival was 92% and only 5.5% of patients had evidence of local failure by 5 years.
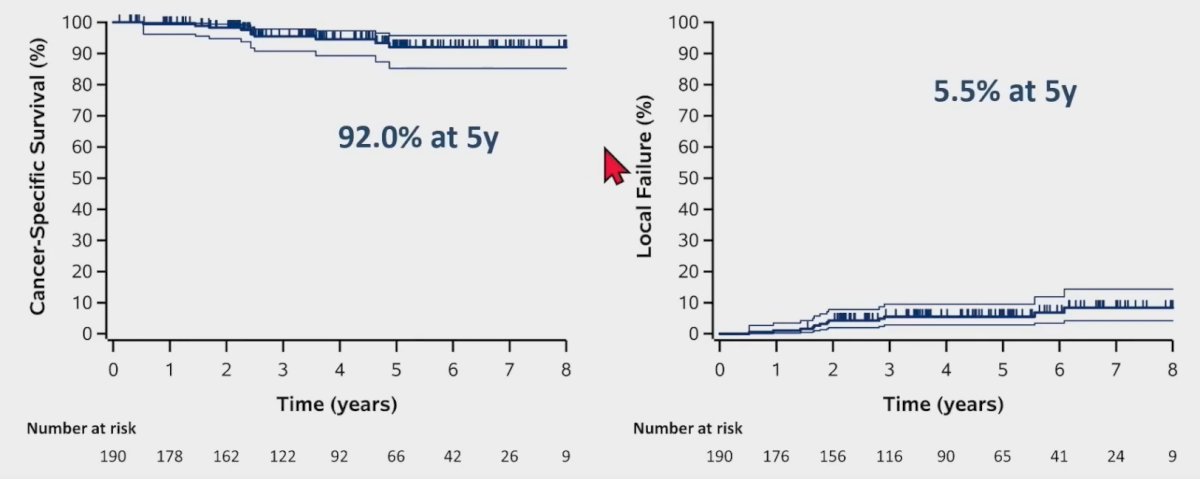
Progression-free survival at 5 years was 64%, and 11% of patients had evidence of distant failure by 5 years.
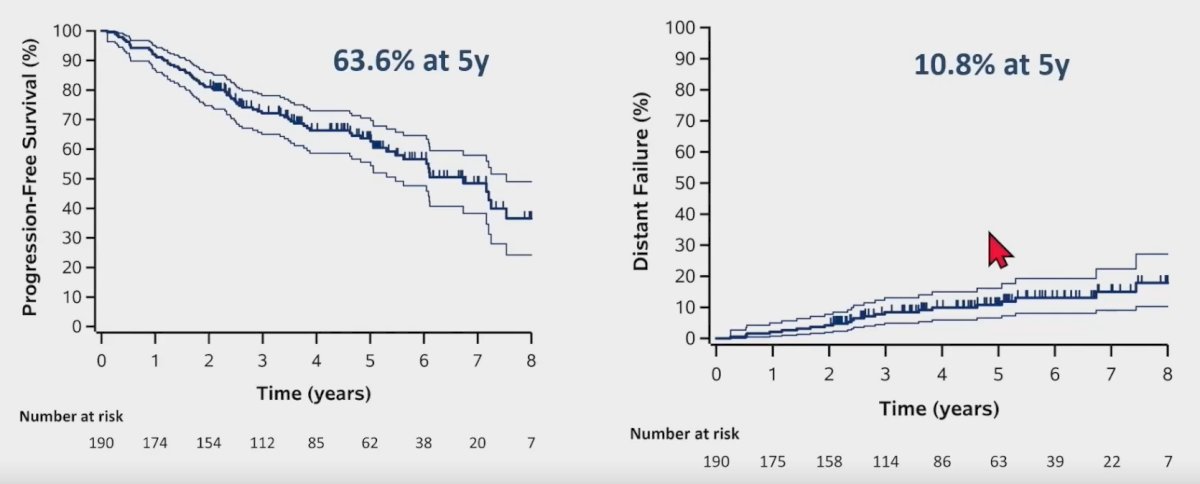
What about long-term renal function? In this cohort of SABR-treated patients, the mean eGFR decline was -10.8 ml/min and -13.5 ml/min at 3 and 5 years, respectively.
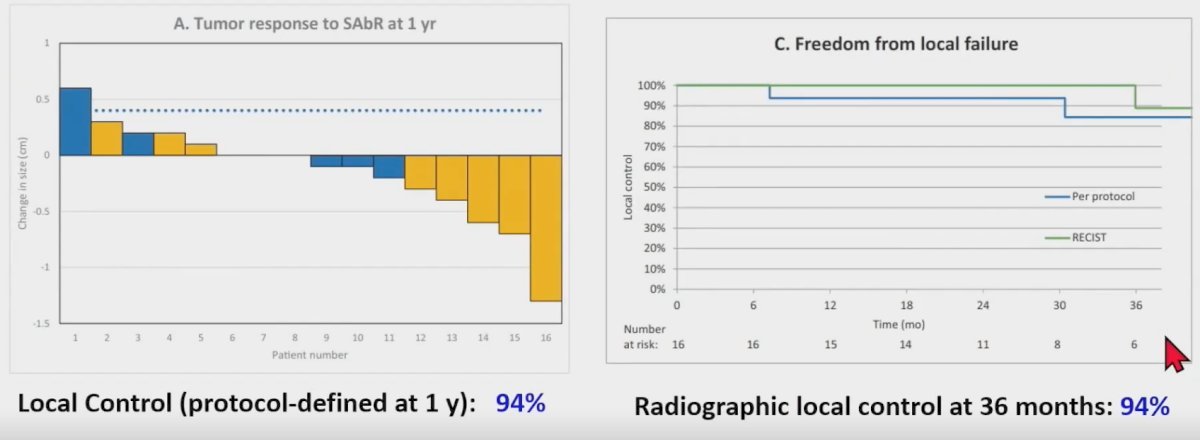
Despite the excellent reported outcomes for SABR, does this treatment modality actually succeed at killing cancer cells? Dr. Correa highlighted a recently published phase II trial by Hannan et al. that evaluated SABR in 16 patients with biopsy-proven RCC ≤5 cm and growing for ≥1 year. Notably, all patients in this trial were evaluated by a multi-disciplinary tumor board and included both operable and non-operable patients. All patients in this trial received linac-based SABR at 36 Gy in 3 fractions or 40 Gy in 5 fractions. The primary endpoint was radiographic response plus pathologic response (1-year post-treatment biopsy).
The median patient age in this trial was 72 years. The initial size was 3.2 cm, and the baseline growth rate was 0.8 cm/year. 11/16 patients (69%) had clear cell histology. This trial met its primary endpoint with a protocol-defined 1-year local control rate of 94%. The 36 months radiographic local control rate was similarly 94%.
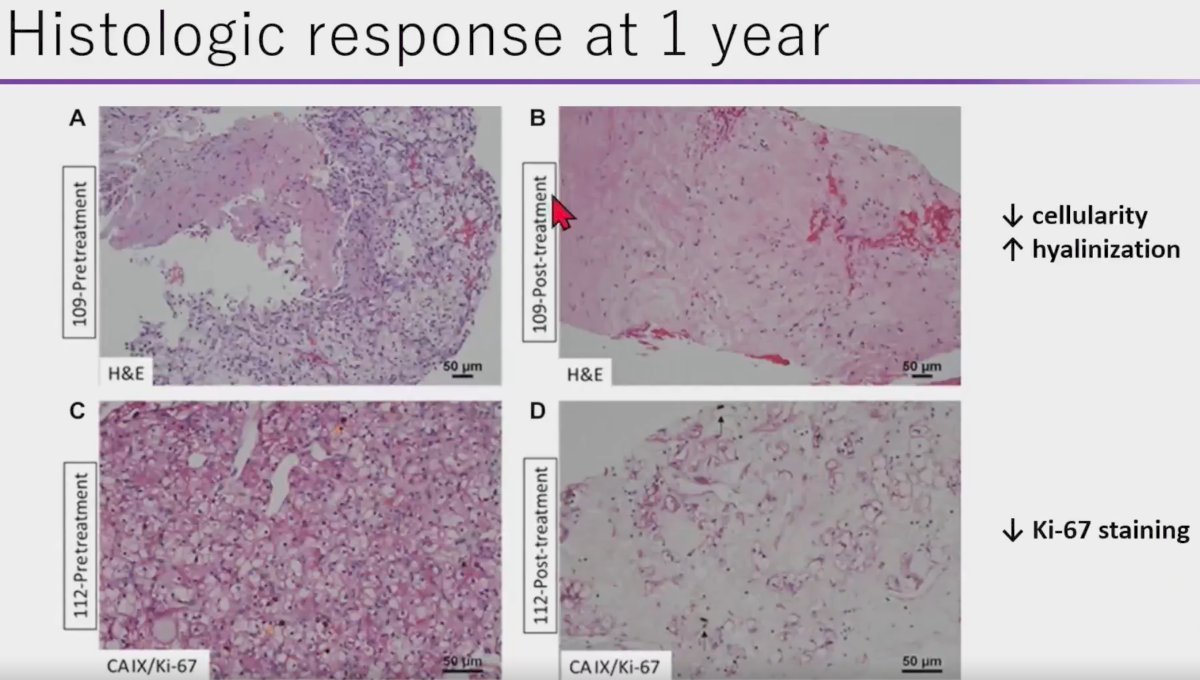
Histopathologic analysis of pre- and post-treatment biopsy specimens demonstrated decreased cellularity, increased hyalinization, and decreased Ki-67 staining (marker of cellular proliferation) in the SABR treated specimens. As such, Dr. Correa argued that a substantial proportion of ‘positive’ biopsies following SABR performed within a short interval of 1 year may actually be false positives. SABR induces infiltration of host immune cells and causes catastrophic DNA damage that may lead to a slow, protracted radiographic response. In this setting, senescent cells persist as non-replicative, terminally differentiated, and quiescent cells eventually re-attempt the cell division cycle, given that RCC is not a rapidly proliferating tumor.
Dr. Correa concluded his presentation as follows:
- Radiotherapy for RCC has experienced a technological renaissance through SABR
- Prospective trials demonstrate efficacy, safety, and low toxicity of SABR for RCC
- Despite ‘false positive’ biopsies, which should not be part of routine clinical practice, in his opinion
- Long-term, multicenter iROCK data suggest favorable oncological outcomes persist up to 5 years
- Including larger T1b tumors
- Prospective, multicenter clinical trials in this disease space are ongoing, including FASTRACK II and RADSTER
Written by: Rashid K. Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 American Society for Therapeutic Radiation Oncology (ASTRO) 65th Annual Meeting held in San Diego, CA between October 1st and 4th, 2023
References:
- Sun M, Abdollah F, Bianchi M, et al. A Stage-for-Stage and Grade-for-Grade Analysis of Cancer-Specific Mortality Rates in Renal Cell Carcinoma According to Age: A Competing-Risks Regression Analysis Eur Urol. 2011;60:1152-9.
- Siva S, Ali M, Correa RJM, et al. 5-year outcomes after stereotactic ablative body radiotherapy for primary renal cell carcinoma: an individual patient data meta-analysis from IROCK (the International Radiosurgery Consortium of the Kidney). Lancet Oncol. 2022;23(12):1508-16.
- Hannan R, McLaughlin MF, Pop LM, et al. Phase 2 Trial of Stereotactic Ablative Radiotherapy for Patients with Primary Renal Cancer. Eur Urol. 2023;84(3):275-86.


