The 2023 ASTRO annual meeting included a session on clinical trials in prostate cancer, featuring a presentation by Dr. Tamim Niazi discussing results of a phase 3 study of hypofractionated, dose escalation radiotherapy versus conventional pelvic radiation therapy followed by high dose rate brachytherapy boost for high risk prostate cancer. The low a\ß ratio of 1.2-2 for prostate cancer suggests high radiation-fraction sensitivity and predicts a therapeutic advantage of lager fraction size.
Dr. Niazi and colleagues have recently shown (PCS5) that high risk prostate cancer patients can safely and effectively be treated with moderate hypofractionated radiation therapy.1 To date, there has been no phase-III randomized clinical-trial comparing moderately hypofractionated radiation therapy with EBRT and HDR boost. At the 2023 ASTRO annual meeting, Dr. Niazi reported the acute safety of EBRT + HDR boost compared to moderate hypofractionated radiation therapy in this phase III Canadian trial.
From January 2015 to June 2022, 308 high-risk localized prostate cancer patients were randomized to receive either hypofractionated radiation therapy or EBRT + HDR boost. All patients received neoadjuvant, concurrent, and long-term adjuvant ADT. EBRT + HDR boost consisted of 46 Gy in 2 Gy per fraction to the pelvis and a 15 Gy in one fraction HDR boost within 3 weeks of EBRT. Hypofractionated radiation therapy include concomitant dose escalation of 68 Gy in 2.72 Gy per fraction to the prostate, and 45 Gy in 1.8 Gy per fraction to the pelvic lymph-nodes. The primary endpoint for PCS6 was acute/delayed toxicity, and secondary endpoints include overall survival, prostate cancer specific mortality, distant metastasis free survival, biochemical failure free survival, and EPIC quality of life. The trial schema is as follows: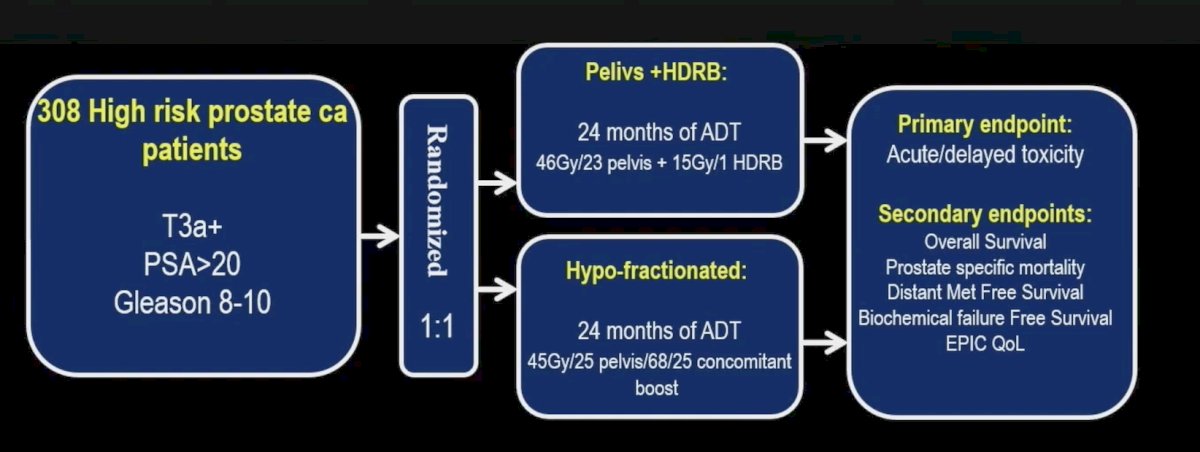
Among the included patients, 153 received hypofractionated radiation therapy and 144 EBRT + HDR boost. The remainder either withdrew from the study or were treated with standard (2 Gy per fraction) fractionation for technical reasons. The baseline characteristics for these patients is as follows: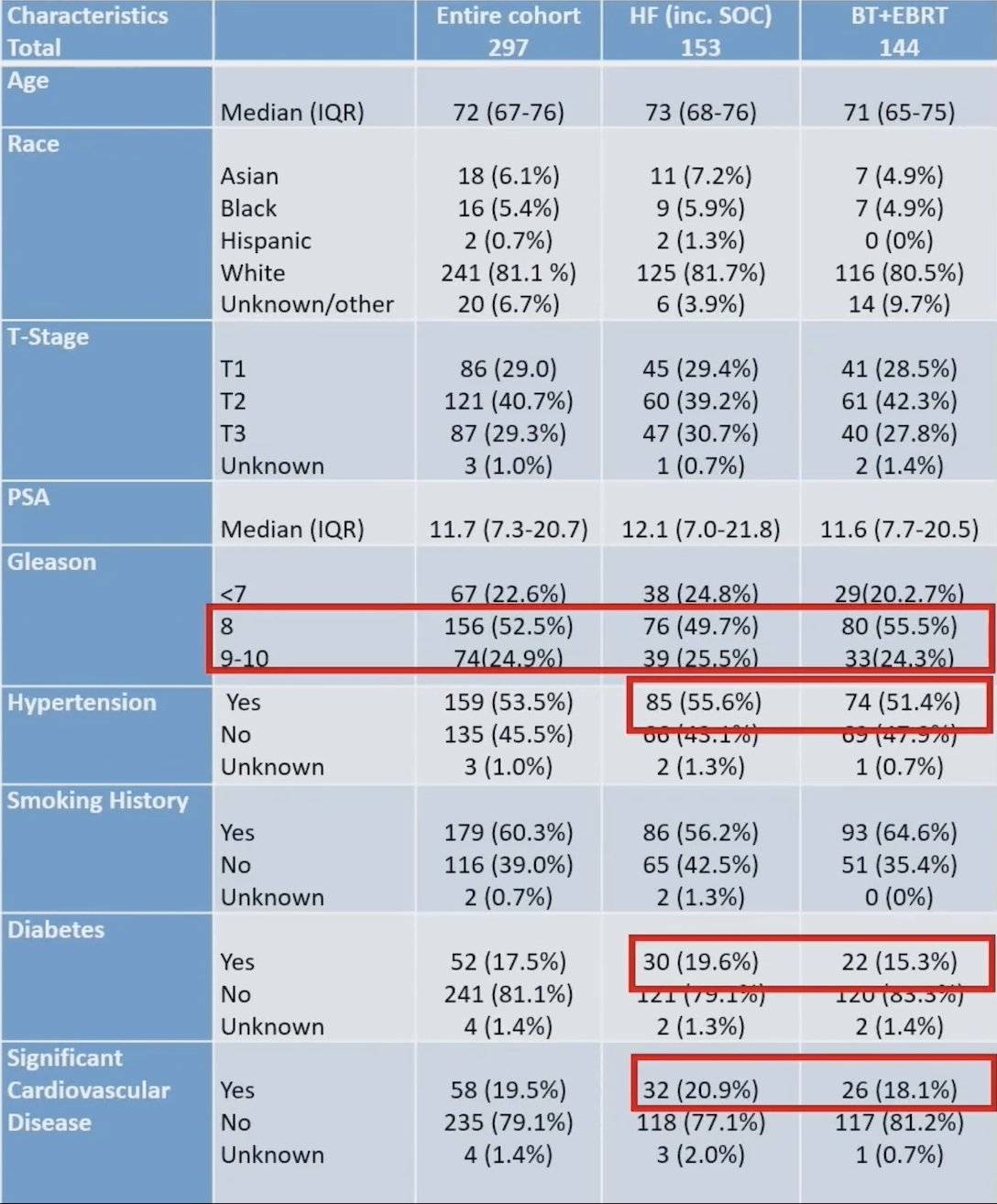
In both intention to treat and as treated analysis, using log-Rank, there were more grade 1 or worse (G1+) acute gastrointestinal and genitourinary events and more G2+ acute gastrointestinal events in the hypofractionated radiation therapy than EBRT + HDR boost. As treated analysis, the acute G1+ and G2+ gastrointestinal events were 92 vs 77 (60.1% vs. 53.5%; p < 0.017) and 21 vs 10 (13.7% vs. 6.9%; p = 0.052), respectively for hypofractionated radiation therapy and EBRT + HDR boost. Similarly, the G1+ acute genitourinary events were 123 vs. 101 (80.4% vs.70.1%; p<0.001) respectively for hypofractionated radiation therapy and EBRT + HDR boost.
The acute gastrointestinal toxicity in the intention to treat analysis is as follows: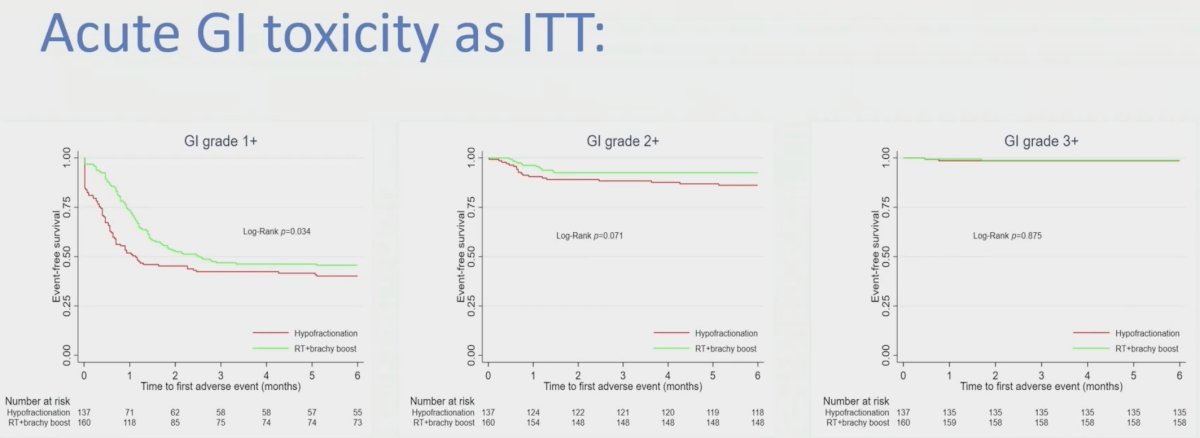
The acute gastrointestinal toxicity in the as treated analysis is as follows: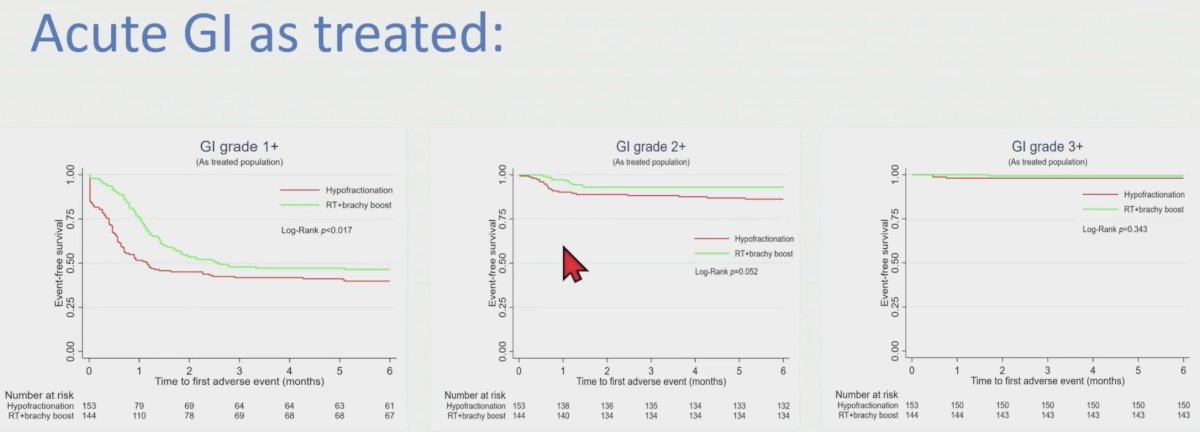
The acute genitourinary toxicity in the as treated analysis is as follows:
There were only four G3 gastrointestinal and one G3 genitourinary acute toxicities in both arms. No grade 4-toxicities were reported.
Dr. Niazi concluded his presentation discussing results of a phase 3 study of hypofractionated, dose escalation radiotherapy versus conventional pelvic radiation therapy followed by high dose rate brachytherapy boost for high risk prostate cancer with the following take-home points:
- This is the first study of EBRT + HDR boost compared to moderate HF dose escalated radiotherapy in high-risk prostate cancer patients treated with long-term ADT and pelvic RT
- With a minimum of 6 months follow-up, both treatment approaches are well-tolerated
- EBRT + HDR boost carries less G2+ gastrointestinal and G1+ genitourinary acute toxicities
- Delayed toxicity and disease outcomes are to follow
Presented by: Tamim Niazi, MD, McGill University Health Centre, Montreal, Quebec, Canada
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2023 American Society of Radiation Oncology (ASTRO) Annual Meeting, San Diego, CA, Sun, Oct 1 – Wed, Oct 4, 2023.
References:
- Niazi T, Nabid A, Malagon T. et al. Dose escalation radiotherapy for high-risk prostate cancer: the safety analysis of the Prostate Cancer Study-5 (PCS-5) a GROUQ led phase III trial. Int J Radiol Oncol Biol Phys. 2023.05.014.


