(UroToday.com) The 2023 American Society for Radiation Oncology (ASTRO) 65th Annual Meeting held in San Diego, CA was host to a plenary session, with Dr. Alejandro Berlin delivering the discussant following Dr. von As’ earlier presentation of the PACE-B study results.
Dr. Berlin began his presentation by discussing how the radiation oncology field ‘got’ to PACE-B, contextualizing this within the history of prostate cancer risk stratification and treatment. Over the last century, radiation technologies have evolved to allow for precise defining and co-localization of targets and therapeutic dose distribution.

This evolution coincided with improved risk stratification of prostate cancer patients using clinicopathologic variables and biomarkers.
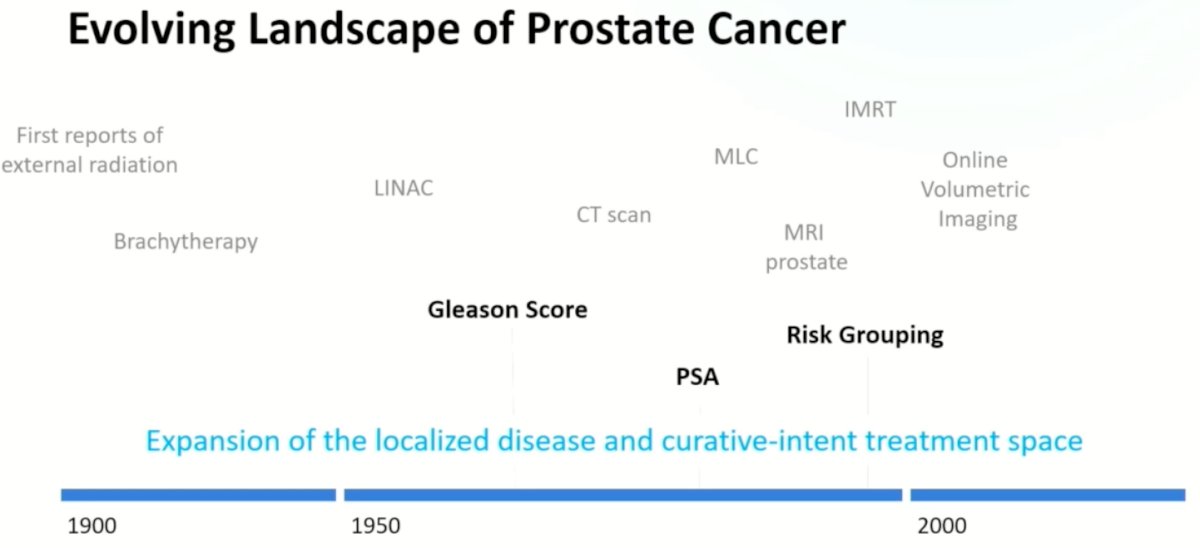
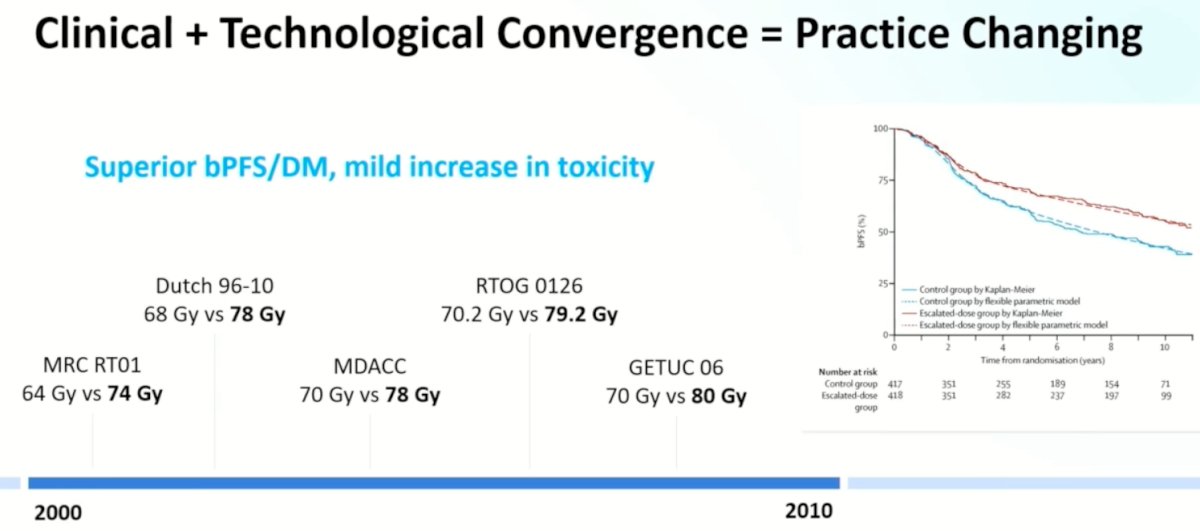
These clinical and technological advances ‘converged’ leading to practice changing trials that demonstrated that higher doses of radiation (74 to 80 Gy) were associated with superior biochemical progression-free survival and distant metastatic rates, at the cost of a mild increase in the toxicity profile.
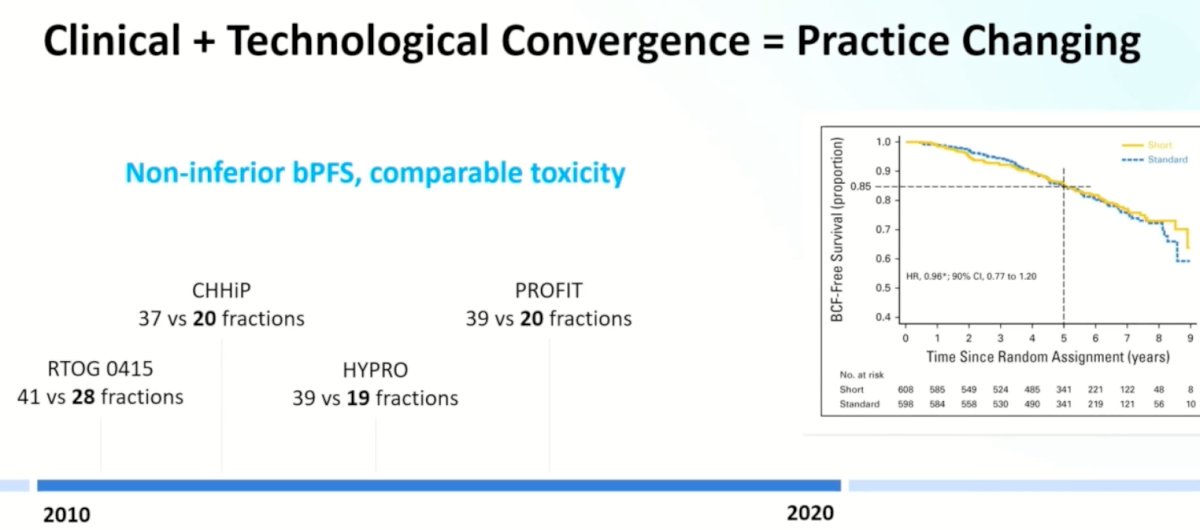
The next step was to ‘compress’ treatment fractions from roughly 39 sessions to 19-20 fractions (i.e., conventional to moderate hypofractionation) with 4 trials demonstrating non-inferior biochemical failure outcomes, with a comparable toxicity profile.
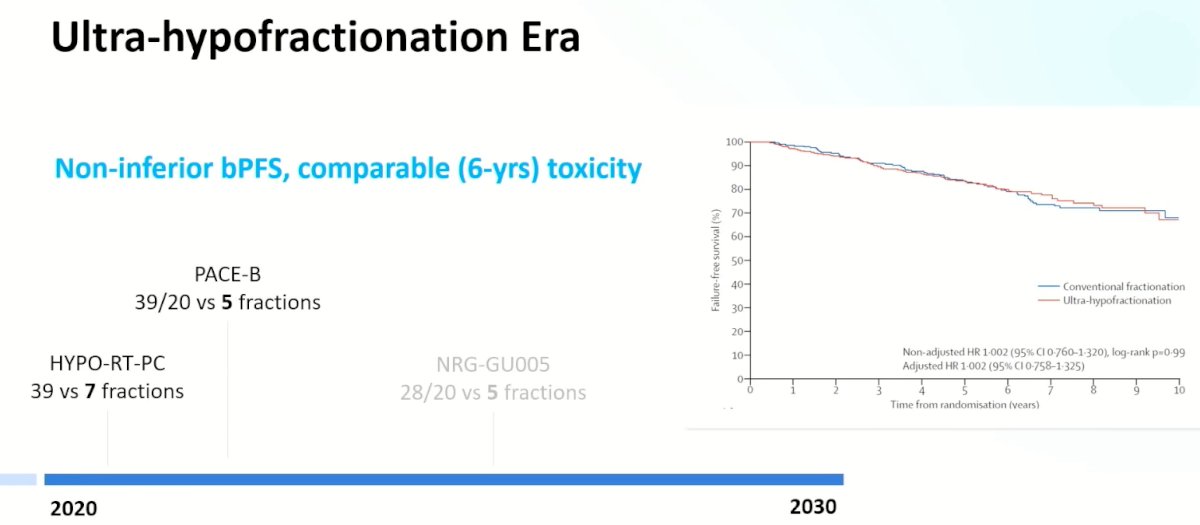
The next step was to evaluate ultra-hypofractionation with radiotherapy administered in 4 to 5 fractions.

This has led us to the ‘ultra-hypofractionation’ era with the PACE-B trial demonstrating that ultra-hypofractionation for patients with predominantly intermediate-risk, clinically localized prostate cancer was non-inferior to conventional radiotherapy for the primary outcome of biochemical or clinical progression-free survival. This trial adds to the growing body of evidence for ultra-hypofractionation with the Scandinavian phase III HYPO-RT-PC trial demonstrating that ultra-hypofractionation was non-inferior to conventionally fractionated radiotherapy.1
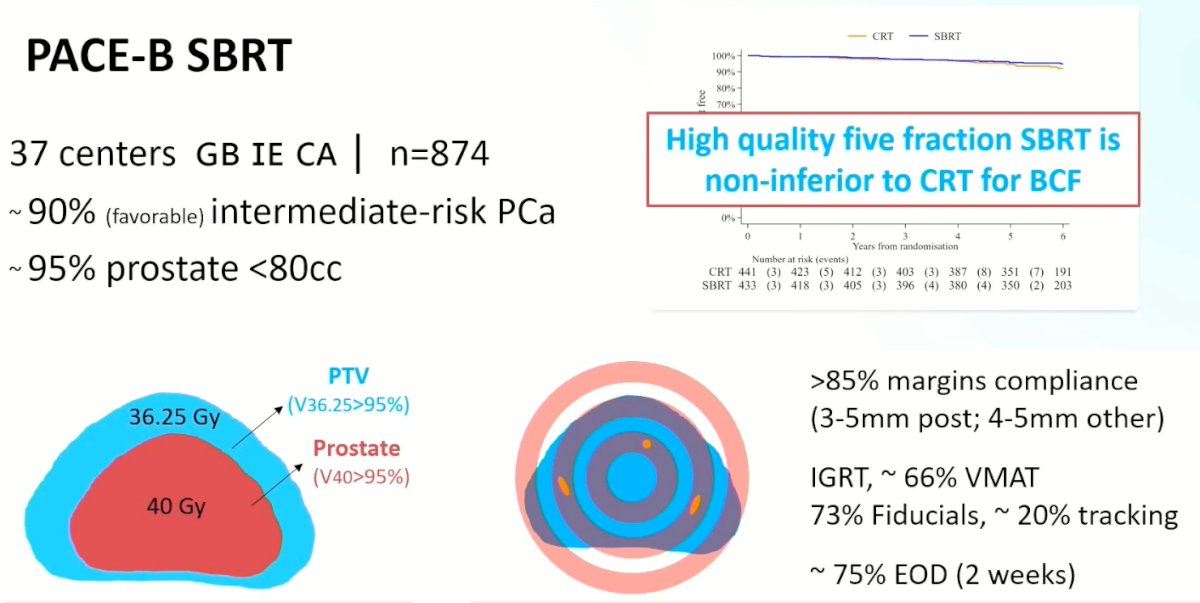
What are the questions that PACE-B addresses? Dr. Berlin provided a high-yield overview of the recently presented PACE-B trial. This trial included 874 patients from 37 centers in the UK, Ireland, and Canada. 90% of included patients had intermediate (mostly favorable) risk disease, and 95% of patients had a prostate <80 cc. The radiotherapy dose was administered at 36.25 Gy in 5 fractions, with a clinical target volume (CTV) of 40 Gy. Dr. Berlin highlighted that this was a ‘high quality’ radiotherapy trial with >85% margin compliance with narrow margins of 3-5 mm posteriorly. Treatment was mostly administered via image-guided radiotherapy with fiducial markers used in 73% of cases for targeting. ~75% of treatments were administered every other day, thus allowing for treatment completion within 2 weeks. Ultimately, this trial demonstrated that high-quality SBRT administered in 5 fractions is non-inferior to conventional radiotherapy for biochemical failure.
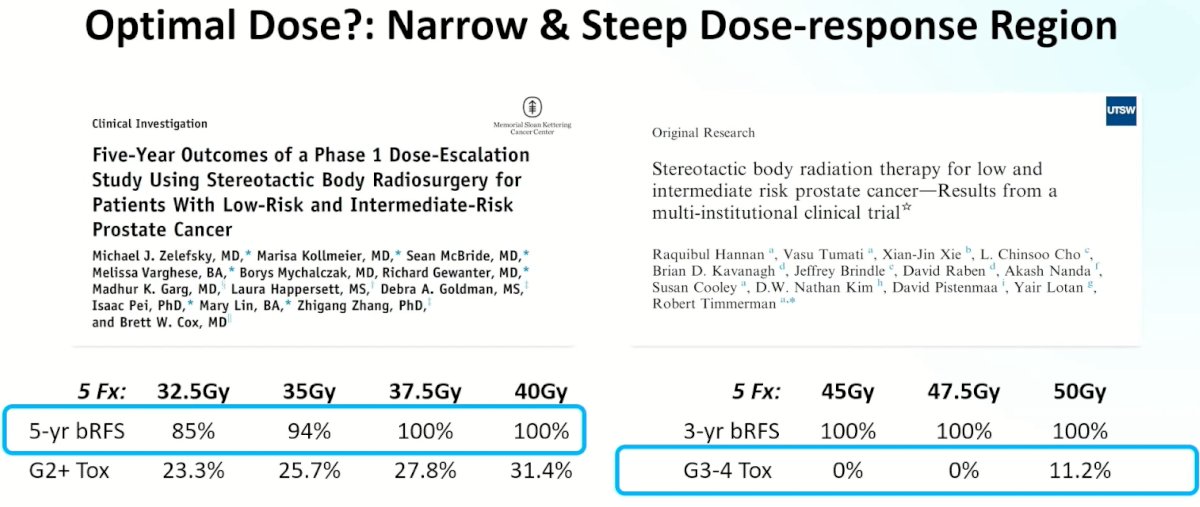
Given these results, and contextualizing this within the current body of evidence, what is the optimal radiotherapy dose? Based on study results from the Memorial Sloan Kettering group, it appears that 5-year biochemical recurrence-free survival outcomes progressively improve up to a dose of 40 Gy with acceptable toxicity profiles. However, as subsequently demonstrated by data from the UT Southwestern group, there does not appear to be additional oncologic benefit for doses >40 Gy, with a significant increase in the toxicity profile.
When comparing outcomes across trials for ultra-hypofractionation, it is important to be aware of the ‘prescription heterogeneity’ with different nominal treatment doses permitted and variations in the allow for targeting of hot spots with the PACE-B trial, for example, allowing for up to 132% dose-escalation, compared to up to 107% in the recently completed NRG-GU005 trial.

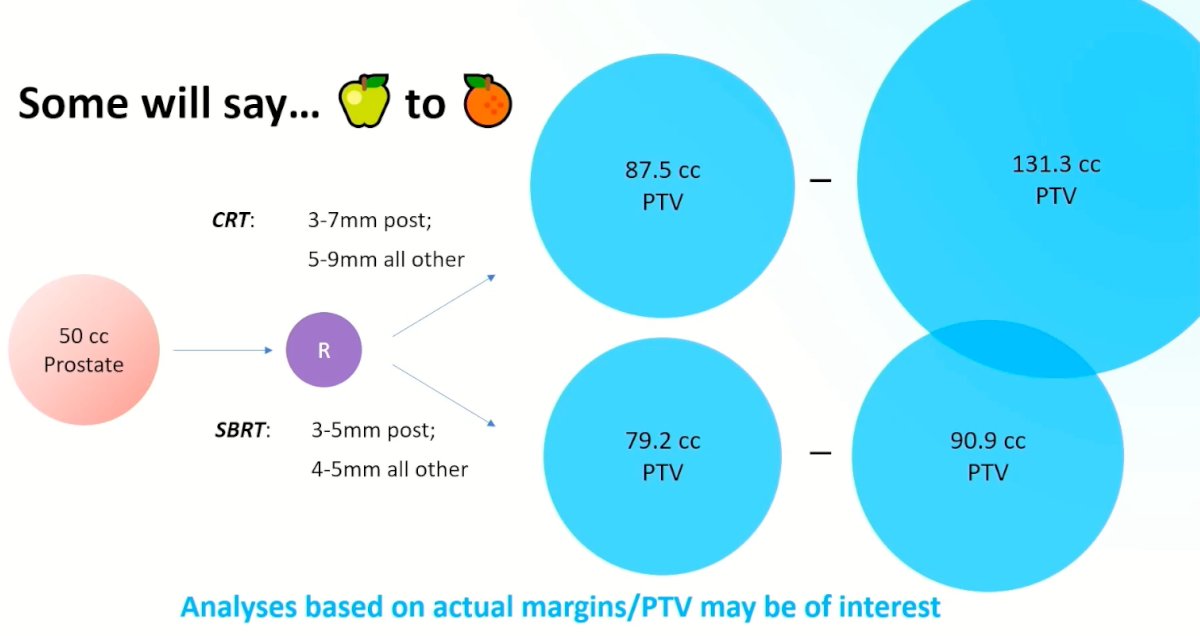
One potential criticism of the PACE-B trial is that the margins criteria were different for patients in the conventional and SBRT arms. In the conventional arm, 3-7 mm posterior margin with 5-9 mm margins elsewhere were allowed. This was in comparison to narrower margins for the SBRT treated patients (3-5 mm posterior, with 4-5 mm for all others). This has important consequences for the ensuing prostate target volume (PTV), with larger PTVs for patients in the conventional arm. As such, Dr. Berlin noted that we await analyses based on actual margins/PTV, which will be of interest.\
However, Dr. Berlin argued that changes in margin status with narrower margins are a feature of evolving technologies, and as such, leveraging the benefits of these technologies with narrow margin allowances with potential subsequent reductions in adverse events is a feature that should be embraced and not criticized. This is exemplified by the recently published MIRAGE trial that compared CT- and MRI-guided SBRT. Patients treated with MR-guidance required a 2 mm margin, compared to 4 mm with CT-guided treatments. This was reflected in a reduction of the proportion of patients with grade 2 or worse GU toxic effects in the MRI-guided SBRT group (24% versus 43%).2

With the presentation of the PACE-B results, what is the current state of ultra-hypofractionated radiotherapy?
- 5 – 7 fraction schedules are a standard-of-care option for localized (intermediate-risk) prostate cancer
- Ultra-hypofractionation is endorsed by all relevant guidelines, increasingly implemented, and practiced
- Ultra-hypofractionation is less forgiving: need to be ‘technically wary’ to achieve the presented results
- Studies necessary to inform and potentially reduce the variability in practices are needed (e.g., margins, dosimetric goals, dose heterogeneity, treatment schedule [daily versus every other day versus weekly], etc.)
- PACE-B is not directly generalizable (albeit informative) to patients in which ADT or pelvic radiotherapy is required
What about the future? Highlighted by recent data from PACE-A and ProtecT, it is clear that the oncologic efficacy of radiotherapy is equivalent to that of surgery. Improving on local control rates compared to surgery may be difficult. As such, to make SBRT the preferred treatment in the future, the focus may need to shift to minimizing the toxicity profile of this treatment. Several ongoing initiatives to reduce radiotherapy-related toxicity include:
- Integrated boost with isotoxic approaches to surrounding organs
- Incorporation of MR gating during delivery
- Rectal spacers
- Selective de-escalation based on the surrounding anatomy
There are also ongoing phase II studies that are evaluating further reductions in the number of treatment fractions. Following the presentation of PACE-B, regimens with <5 fractions should be considered investigational. Whether SBRT administered in 2 fractions will prove to be broadly applicable and safe is yet to be determined.
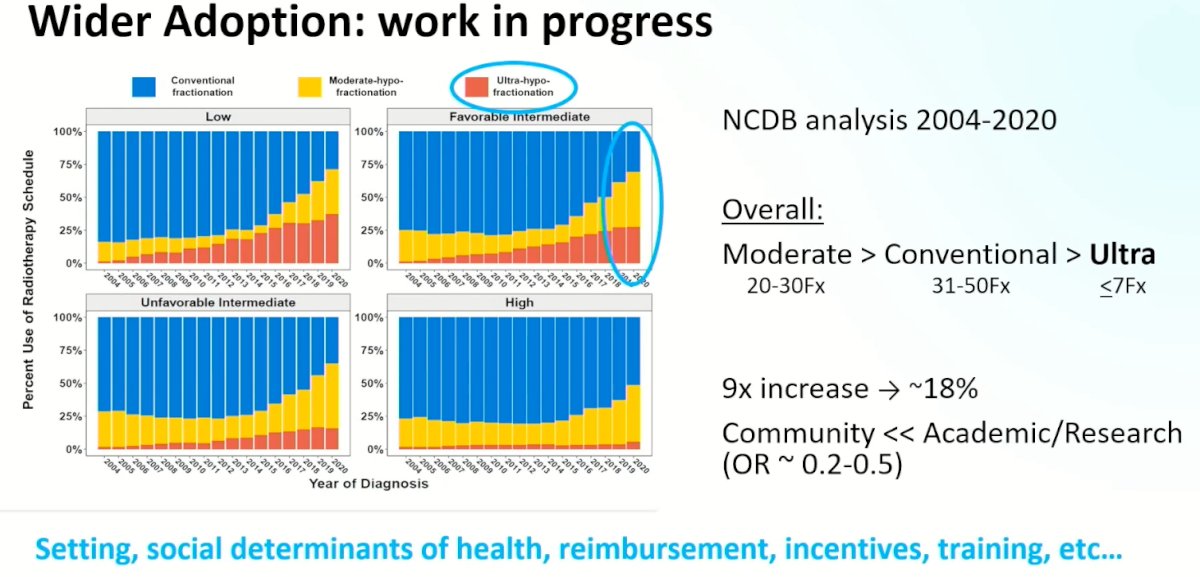
Ultimately, further efforts are needed to improve the adoption of these technical advances in clinical practice. A recent analysis from the National Cancer Database (NCDB) between 2004 and 2020 has demonstrated that the use of moderate hypofractionation is increasing, whereas the use of ultra-hypofractionation is lagging behind, particularly in the community setting. This highlights the importance of factors such as treatment location, social determinants of health, reimbursement incentives, and training for clinical implementation in this setting.
Dr. Berlin concluded his presentation by noting that PACE-B is a major step in the field with the following take home messages:
- The return on investment for ‘physical’ precision has likely plateaued
- Further dose increases is now debatable
- The shifting challenge is to reduce toxicities and improves systemic and biologic precision
Written by: Rashid K. Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 American Society for Therapeutic Radiation Oncology (ASTRO) 65th Annual Meeting held in San Diego, CA between October 1st and 4th, 2023
References:
- Widmark A, Gunnlaugsson A, Beckman L, et al. Ultra-hypofractionated versus conventionally fractionated radiotherapy for prostate cancer: 5-year outcomes of the HYPO-RT-PC randomised, non-inferiority, phase 3 trial. Lancet. 2019;394(10196):385-95.
- Kishan AU, Ma TM, Lamb JM, et al. Magnetic Resonance Imaging-Guided vs Computed Tomography-Guided Stereotactic Body Radiotherapy for Prostate Cancer: The MIRAGE Randomized Clinical Trial. JAMA Oncol. 2023;9(3):365-73.


