(UroToday.com) The 2023 ASTRO annual meeting included a session on oligometastatic prostate cancer, featuring a presentation by Dr. Chad Tang discussing preserving quality of life in the “chronic disease” state of oligometastatic prostate cancer. Dr. Tang started by highlighting his learning objectives of (i) improving understanding of trials supporting metastasis directed therapy for oligometastatic prostate cancer, and (ii) understanding endpoints utilized for trials investigating metastasis directed therapy for oligometastatic prostate cancer. It is important to note that metastatic prostate cancer frontline therapies are associated with:
- Toxicity: the current standard of care of ADT + second generation androgen receptor blockers is associated with significant grade 3+ toxicity
- Financial cost: systemic therapy costs $100-200K per year and require regular injections
- Efficacy: >3-5 year progression free survival rates
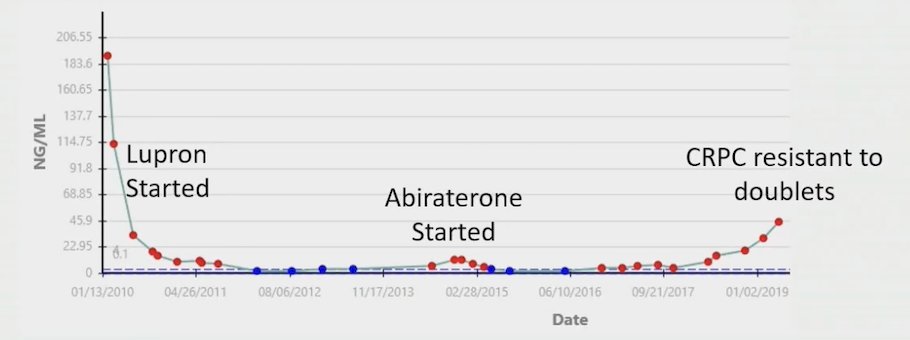
Dr. Tang then discussed a case of a patient diagnosed in 2001 with high risk prostate cancer treated with radical prostatectomy. In 2010, his PSA rose to 191.2 ng/mL, and a CT and bone scan showed extensive pelvic lymphadenopathy, with left hydroureter and a right iliac bone metastasis; at this point, he was started on ADT. In 2014, he was CRPC with another PSA rise + radiographic progression and he was started on abiraterone. In 2019, his PSA had increased to 45.1 ng/mL and he was referred to Dr. Tang. As follows is this patient’s timeline:
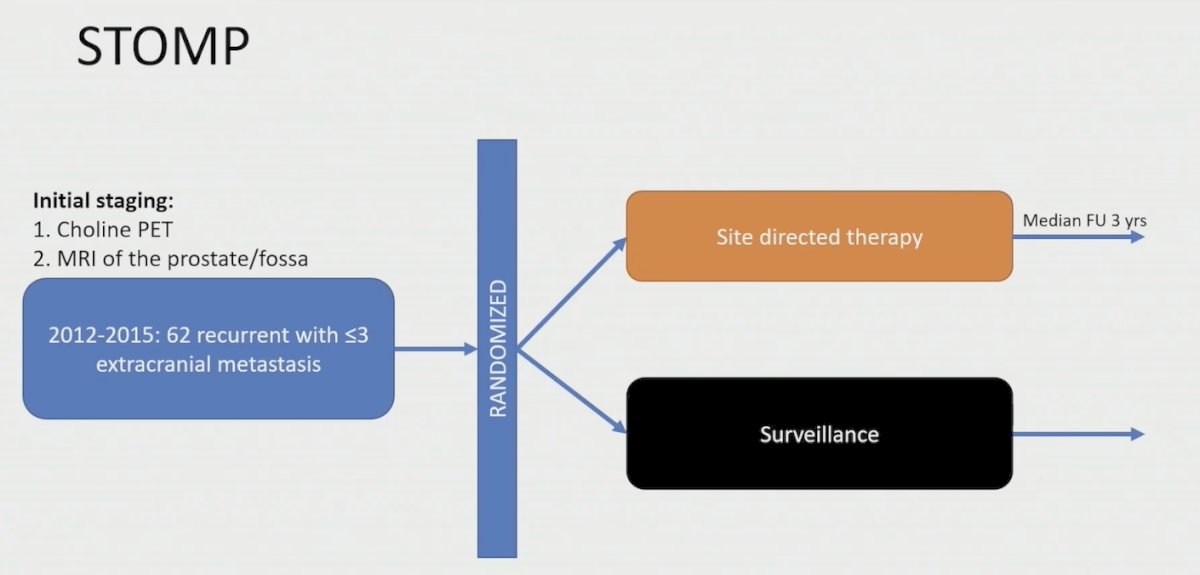
Dr. Tang then discussed the STOMP trial,1 which included 62 patients randomized to either surveillance or metastasis-directed therapy of all detected lesions (surgery or stereotactic body radiotherapy), with a primary endpoint of ADT-free survival:
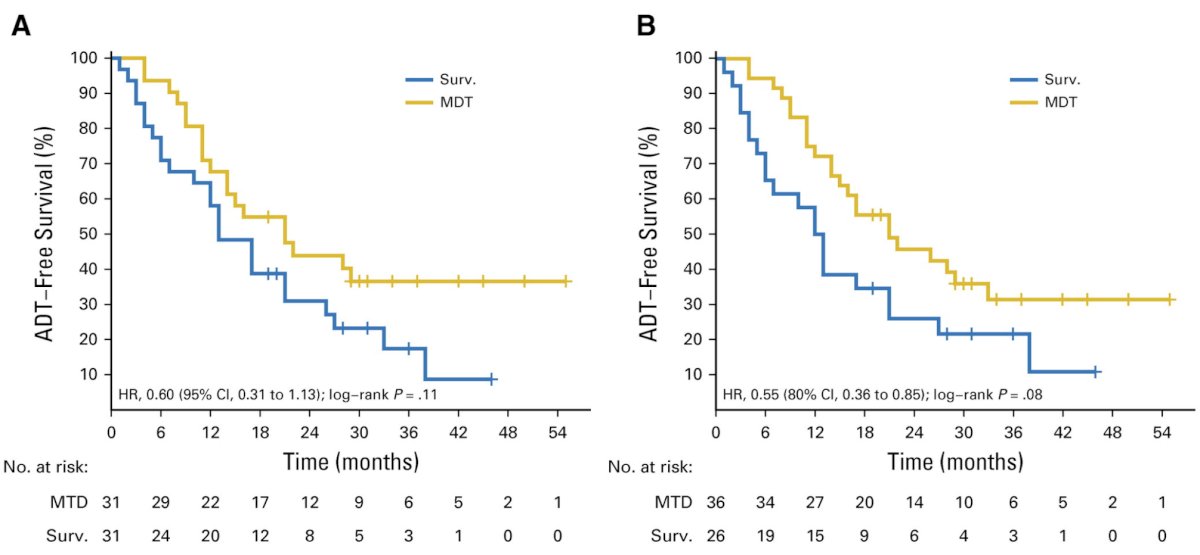
At a median follow-up time of 3 years (IQR 2.3-3.75 years), the median ADT-free survival was 13 months (80% CI 12 to 17 months) for the surveillance group and 21 months (80% CI 14 to 29 months) for the metastasis directed therapy group (HR 0.60, 80% CI 0.40 to 0.90; log-rank p = 0.11):
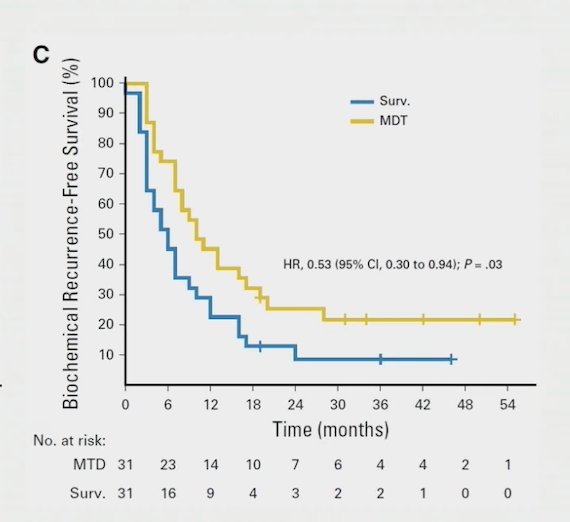
Additionally, patients undergoing metastasis directed therapy had an improvement in biochemical recurrence free survival (HR 0.53, 95% CI 0.30 – 0.94):
Dr. Tang then highlighted the other phase 2 trial in this disease space, the ORIOLE trial,2 which randomized 54 men in a 2:1 ratio to receive stereotactic body radiotherapy or observation. The primary endpoint for this trial was progression at 6 months, defined as a PSA increase, radiographic or symptomatic progression, ADT initiation, or death:
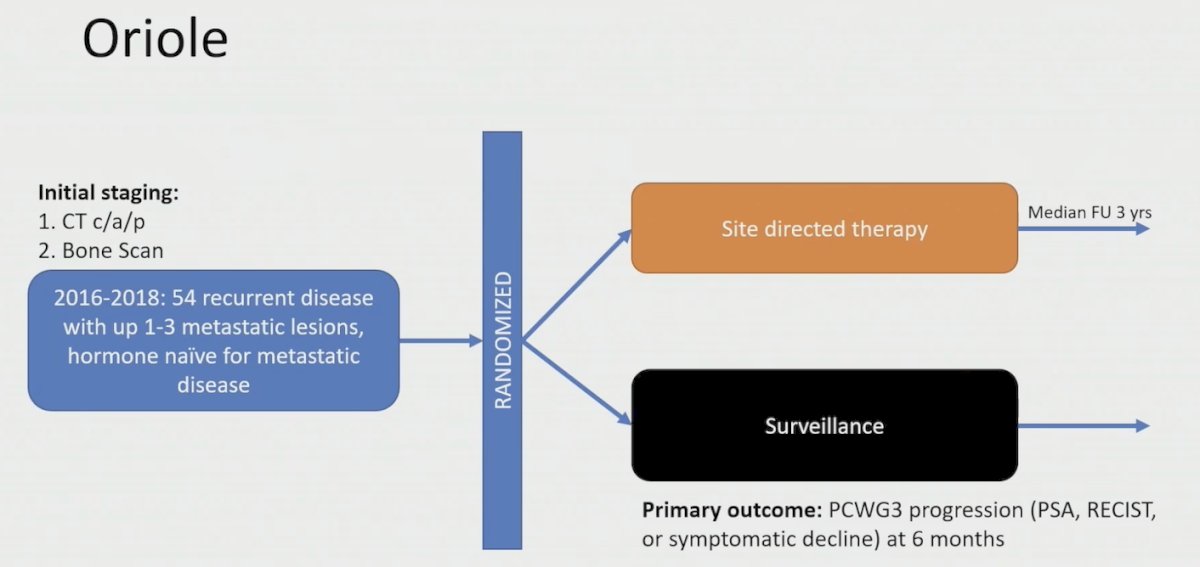
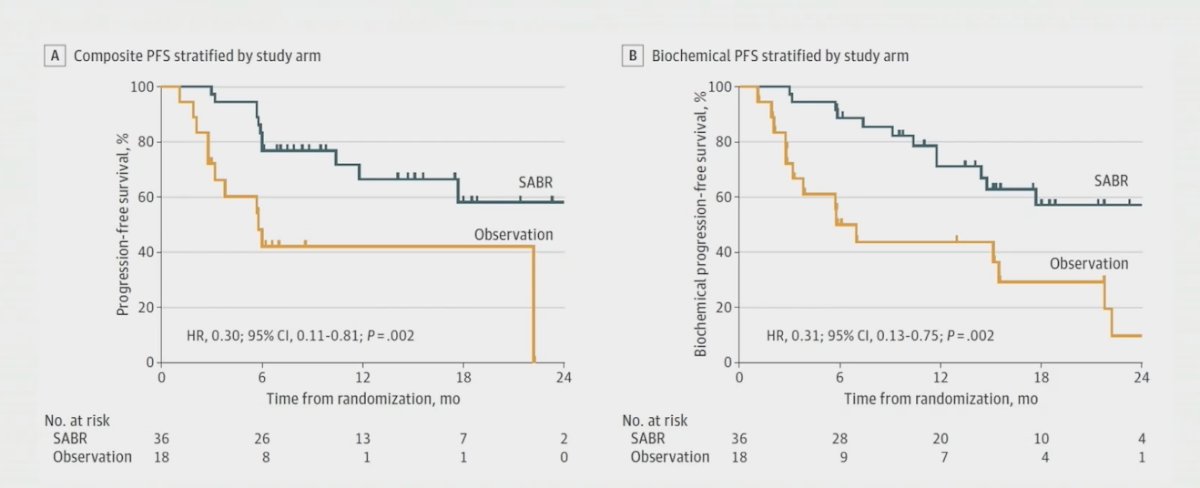
Progression at 6 months occurred in 7 of 36 patients (19%) receiving stereotactic body radiotherapy and 11 of 18 patients (61%) undergoing observation (p = 0.005). Treatment with stereotactic body radiotherapy improved median progression-free survival (not reached vs 5.8 months; HR 0.30, 95% CI 0.11-0.81; p = 0.002). Furthermore, treatment with stereotactic body radiotherapy also improved median biochemical progression-free survival (HR 0.31, 95% CI 0.13-0.70; p = 0.002):
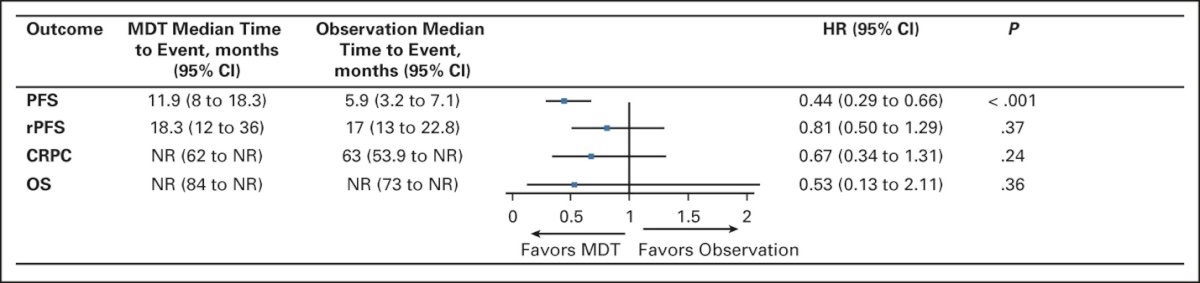
Recently published pooled data from the two trials (STOMP and ORIOLE) demonstrates that metastasis directed therapy in these patients improves progression free survival from 5.9 months (95% CI: 3.2 – 7.1) to 11.9 months (95% CI: 8.0 – 18.3; HR: 0.44, p < 0.001), without any significant improvements seen in radiographic progression-free survival, time to castration-resistant disease, or overall survival:3
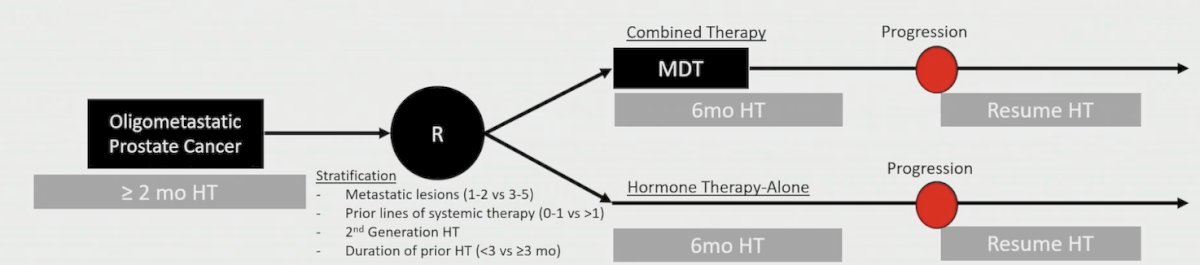
Next, Dr. Tang discussed the EXTEND trial, which was a phase 2 trial assessing the impact of metastasis directed therapy on intermittent hormone therapy for oligometastatic prostate cancer [4]. The major inclusion criteria included (i) <=5 metastases, (ii) >=2 months of prior hormone therapy (either GnRH agonist/antagonist +/- second generation hormone therapy), and (iii) untreated primaries were allowed, but must be treated regardless of randomization. The trial design for EXTEND is as follows:
Among 87 men over a median follow-up of 22.0 months (range: 11.6-39.2), progression free survival was improved in the combined therapy arm (median not reached) compared with the hormone therapy only arm (median, 15.8 months; 95% CI, 13.6-21.2 months; HR 0.25, 95% CI 0.12-0.55):
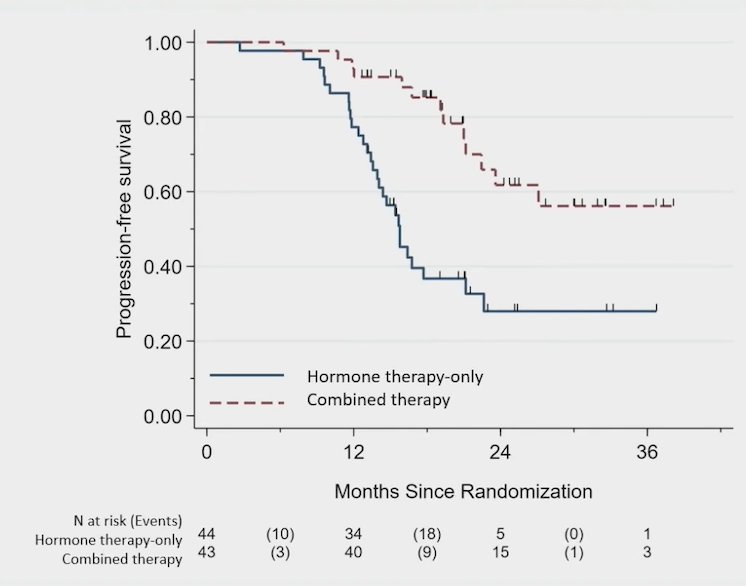
Eugonadal progression free survival was also improved with metastasis directed therapy (median not reached) compared with the hormone therapy only (6.1 months; 95% CI, 3.7 months to NE; HR 0.32, 95% CI 0.11-0.91):

The final trial Dr. Tang discussed was the very recently published ARTO trial.5 For this trial, all patients had oligometastatic CRPC defined as three or fewer nonvisceral metastatic lesions. Patients were randomly assigned 1:1 to receive either abiraterone alone (control arm) or abiraterone with concomitant SBRT to all the sites of disease (experimental arm). The primary endpoint was the rate of biochemical response, defined as a PSA decrease ≥50% from baseline measured at 6 months from treatment start. Complete biochemical response, defined as PSA < 0.2 ng/mL at 6 months from treatment, and progression-free survival were secondary endpoints. The trial design for ARTO is as follows:
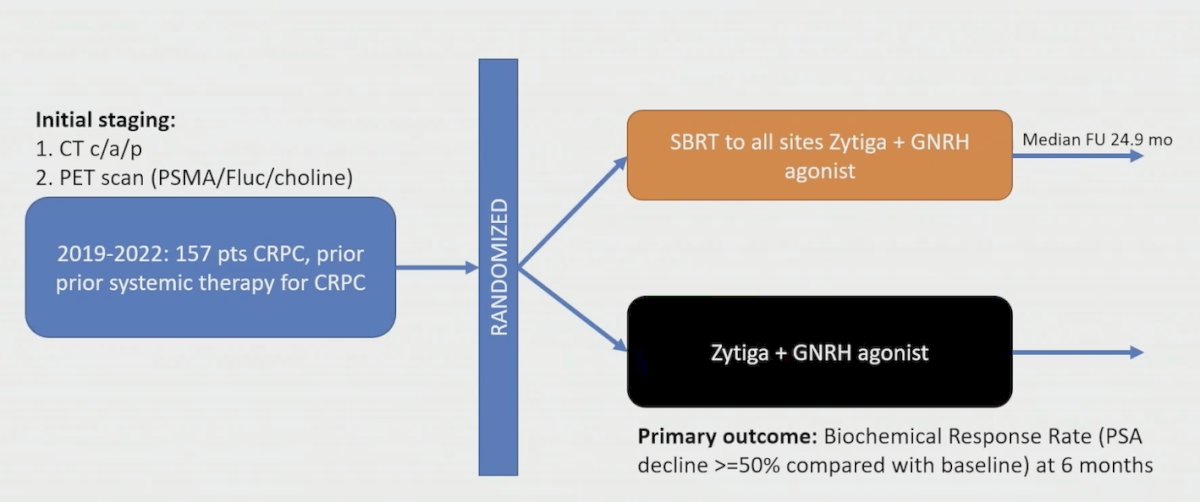
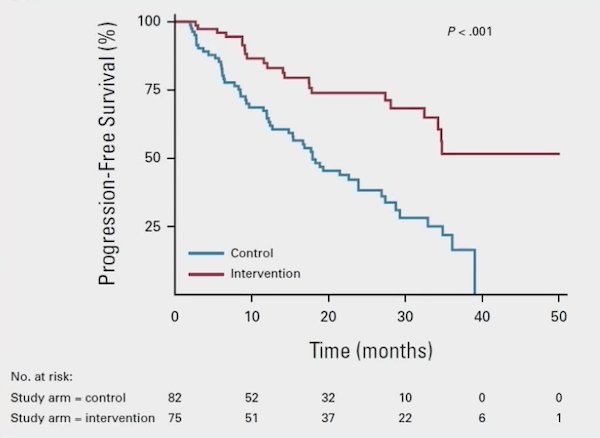
There were 157 men enrolled in ARTO, and biochemical response was detected in 79.6% of patients (92% versus 68.3% in the experimental versus control arm, respectively), with an OR of 5.34 (95% CI 2.05 to 13.88) in favor of the experimental arm. Complete biochemical response was detected in 38.8% of patients (56% versus 23.2% in the experimental versus control arm, respectively), with an OR of 4.22 (95% CI 2.12 to 8.38). Additionally, SBRT resulted in a significant progression free survival improvement, with a HR of 0.35 (95% CI, 0.21 to 0.57) in the experimental versus control arm:
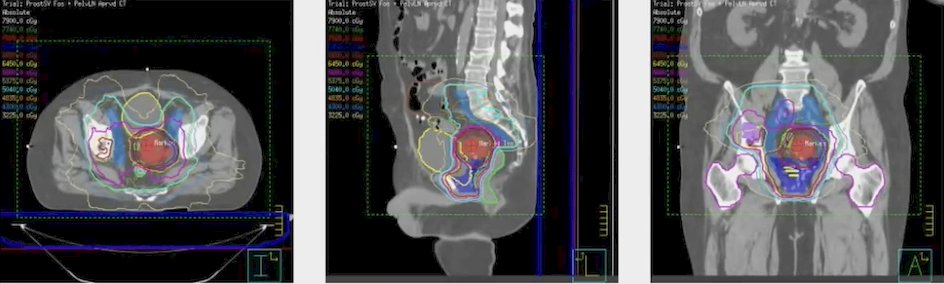
Going back to Dr. Tang’s aforementioned patient, the radiation plan that he completed in 2019 included:
- 50.4 Gy/28 fractions to the elective lymph nodes
- 58.8 Gy/28 fractions to the bone metastases and gross lymph nodes
- 66 Gy/33 fractions to the prostate fossa:
Subsequently, in 2021 he stopped abiraterone, in 2022 he stopped ADT (off all systemic therapies), and in 2023 his PSA has been undetectable.
A second case from Dr. Tang included a 58 year old man treated with a radical prostatectomy in 2012 for localized prostate cancer. In 2014, he had a PSA recurrence and was treated with salvage IMRT, and in 2017 a C-11 choline PET showed common iliac and left internal iliac lymph nodes, as well as a PSA increasing from 0 to 25.4 (started on ADT). In 2018, he was enrolled in the EXTEND intermitted ADT group and he subsequently stopped ADT 6 months later. The patient was randomized to no radiation and in 2019 his testosterone recovered. His last follow-up was in July 2023, with an undetectable PSA and testosterone level of 253 ng/mL.
Dr. Tang concluded this presentation by discussing preserving quality of life in the “chronic disease” state of oligometastatic prostate cancer with the following take-home points:
- Systemic therapy is highly effective for prostate cancer, but indefinite use is associated with substantial toxicities, costs, and detriments in quality of life
- In hormone naïve patients, metastasis directed therapy has shown utility in deferring initiation of hormone therapy (STOMP/ORIOLE)
- In patients on hormone therapy, metastasis directed therapy can facilitate a prolonged ADT-free holiday (EXTEND) or in CRPC time until disease progression (ARTO)
Presented by: Chad Tang, MD Anderson Cancer Center, Houston, TX
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2023 American Society for Therapeutic Radiation Oncology (ASTRO) 65th Annual Meeting held in San Diego, CA between October 1st and 4th, 2023
References:
- Ost P, Reynders D, Decaestecker K, et al. Surveillance of Metastasis-Directed Therapy for Oligometastatic Prostate Cancer Recurrence: A Prospective, Randomized, Multicenter Phase II Trial. J Clin Oncol 2018 Feb 10;36(5):446-453.
- Phillips R, Shi WY, Deek M, et al. Outcomes of Observation vs Stereotactic Ablative Radiation for Oligometastatic Prostate Cancer: The ORIOLE Phase 2 Randomized Clinical Trial. JAMA Oncol 2020 Mar 26;6(5):650-659.
- Deek MP, van der Eecken K, Sutera P, et al. Long-Term Outcomes and Genetic Predictors of Response to Metastasis-Directed Therapy Versus Observation in Oligometastatic Prostate Cancer: Analysis of STOMP and ORIOLE Trials. J Clin Oncol. 2022;JCO2200644.
- Tang C, Sherry AD, Haymaker C, et al. Addition of metastasis-directed therapy to intermittent hormone therapy for oligometastatic prostate cancer: The EXTEND phase 2 randomized clinical trial. JAMA Oncol. 2023 Jun 1;9(6):825-834.
- Fancolini G, Allegra AG, Detti B, et al. Stereotactic body radiation therapy and abiraterone acetate for patients affected by oligometastatic castrate-resistant prostate cancer: A randomized phase II trial (ARTO). J Clin Oncol. 2023 Sep 21;JCO2300985.


