Dr. van As began by highlighting that PACE is an ‘umbrella’ platform of 4 trials (PACE-NODES in higher risk disease not shown in the schematic below), all evaluating radiotherapy to the prostate in patients with localised prostate cancer. Patients with low- or intermediate-risk disease who were considered for surgical intervention were randomized to surgery versus SBRT (PACE-A; presented at GU ASCO 2023), whereas patients who were not considered for surgery were ‘funneled’ to PACE-B, as demonstrated below.
.

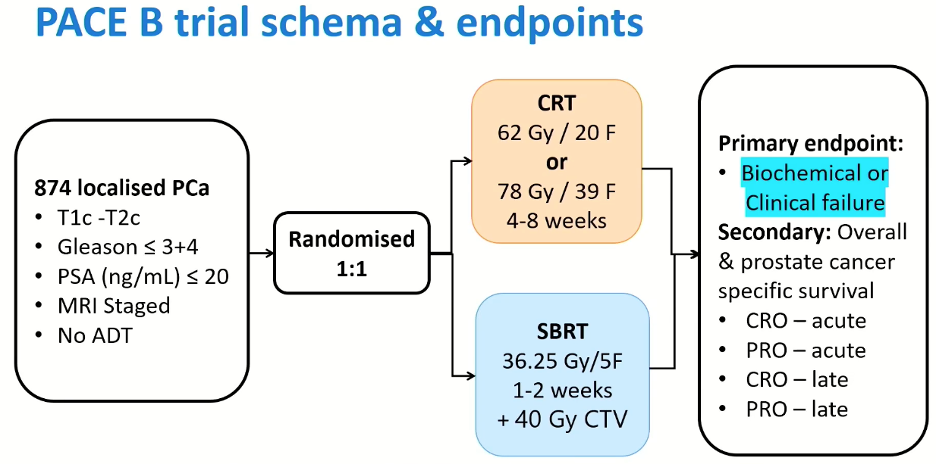
PACE-B included 874 patients with localized prostate cancer meeting the following inclusion criteria:
- cT1c-2c
- Gleason score ≤ 3+4=7
- PSA ≤ 20 ng/ml
- MRI staged
- No ADT
Patients were randomized in a 1:1 fashion to the control arm of conventional fractionation at 78 Gy in 39 fractions given over 4-8 weeks, which was later modified to 62 Gy in 20 fractions following the publication of the CHHiP trial. Patients randomized to the experimental arm received SBRT at 36.25 Gy in 5 fractions over 1-2 weeks with 40 Gy clinical target volume (CTV). The primary endpoint was biochemical or clinical failure, with secondary outcomes of overall and prostate cancer-specific survival, as well as quality of life outcomes.
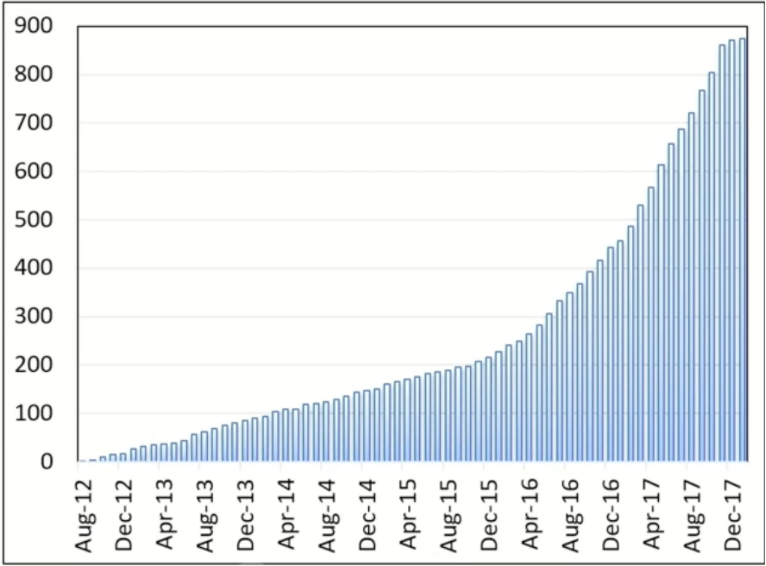
PACE-B was designed as a non-inferiority trial, with 80% power to exclude a critical hazard ratio of 1.45 (1-sided 5% alpha), and required 858 participants. The time point of primary interest was 5 years from randomization. 874 patients were recruited from 37 radiotherapy centers in the UK, Ireland, and Canada between August 2012 and January 2018. The data cut-off was September 11, 2023, and the median follow-up was 74 months.
The baseline patient characteristics were well-balanced between the two treatment arms. The median patient age was 70 years. This trial predominantly included patients with intermediate-risk disease (90%).

There were no significant differences in the primary endpoint of biochemical or clinical failure, as demonstrated in the Kaplan Meier curve below, with freedom from failure rates of approximately 5% in each arm:
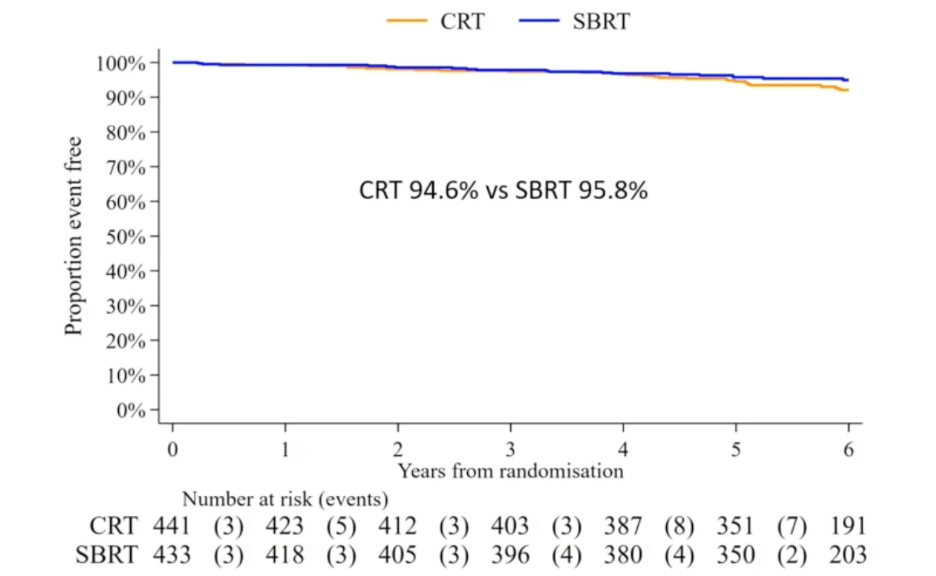
As such, the investigators were able to demonstrate the non-inferiority of SBRT, compared to standard conventionally fractionated radiotherapy.

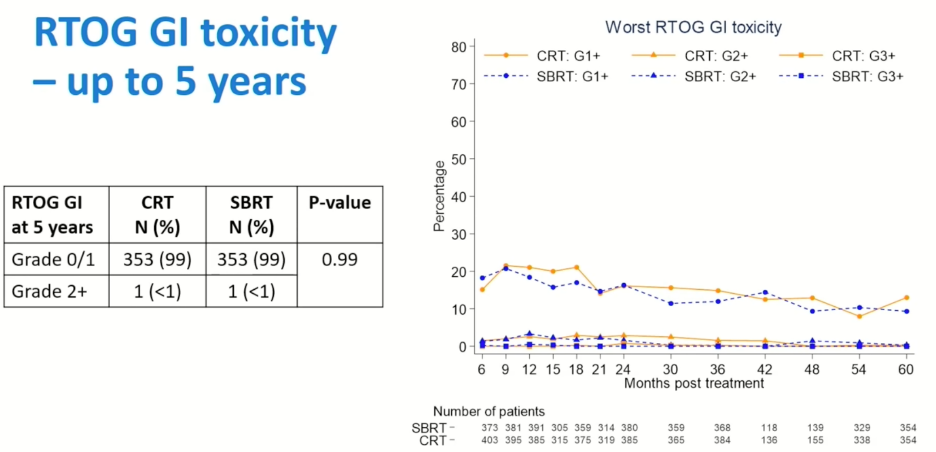
There were no significant differences in 5-year RTOG-defined gastrointestinal (GI) toxicity rates as demonstrated below, with 1 patient in each arm experiencing grade 2 or worse GI toxicity. Similarly, there were no significant differences in Common Terminology Criteria for Adverse Events (CTCAE)-defined GI adverse events.
 Similarly, there were no significant differences in the 5-year rates of genitourinary (GU) toxicity, with grade 2 or worse GU events in 5.4% of SBRT patients and 3.7% of those in the CRT arm (p=0.28). With respect to CTCAE GU toxicity, the rate of grade 2 or worse events appear to be slightly worse in the SBRT arm (8.5% versus 5.9%), although this did not meet statistical significance (p=0.19).
Similarly, there were no significant differences in the 5-year rates of genitourinary (GU) toxicity, with grade 2 or worse GU events in 5.4% of SBRT patients and 3.7% of those in the CRT arm (p=0.28). With respect to CTCAE GU toxicity, the rate of grade 2 or worse events appear to be slightly worse in the SBRT arm (8.5% versus 5.9%), although this did not meet statistical significance (p=0.19).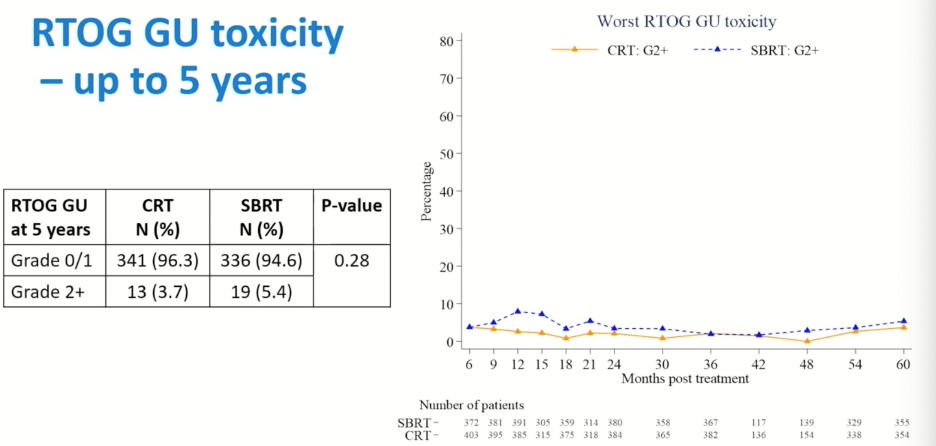
Dr. von As concluded his presentation with the following take home messages:
- Biochemical and clinical failure rates were very low in PACE-B
- SBRT administered at 36.25 Gy in 5 fractions is non-inferior to conventional radiotherapy for patients with intermediate risk, localized prostate cancer
- Toxicity is low for both conventional radiotherapy and SBRT
- SBRT is more convenient for patients and more cost effective for health care providers
- SBRT should be considered a new standard of care in low and favorable intermediate risk prostate cancer
Presented By: Professor Nicholas van As, MD, MB, Consultant Oncologist, The Royal Marsden NHS Foundation Trust, London, UK
Written By: Rashid K. Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 American Society for Therapeutic Radiation Oncology (ASTRO) 65th Annual Meeting held in San Diego, CA between October 1st and 4th, 2023
References:

