(UroToday.com) The 2023 American Society for Radiation Oncology (ASTRO) 65th Annual Meeting held in San Diego, CA between October 1st and 4th, 2023 was host to a session discussing combined modality therapy for lymph node positive prostate cancer. Following an earlier presentation by Dr. Leslie Ballas on post-operative therapy for prostate cancer patients with pathologic node positive disease, Dr. Omar Mian discussed optimizing multimodality therapy for clinically node positive prostate cancer.
Dr. Mian began by highlighting that approximately 13% of newly diagnosed prostate cancer cases are node-positive, significantly contributing to the total number of deaths from prostate cancer. With the widespread use of PSMA PET for staging, the number of cN1 patients will likely increase. Current data supports the use of early ADT in men with N+ prostate cancer:
- Post-operative setting in patients with pN+ disease: Based on results of RTOG ECOG 3886 published by Messing et al. in 20061
- Concurrently with radiotherapy in patients with cN+ disease based on the results of RTOG 85-31 published by Pilepich et al. in 20052
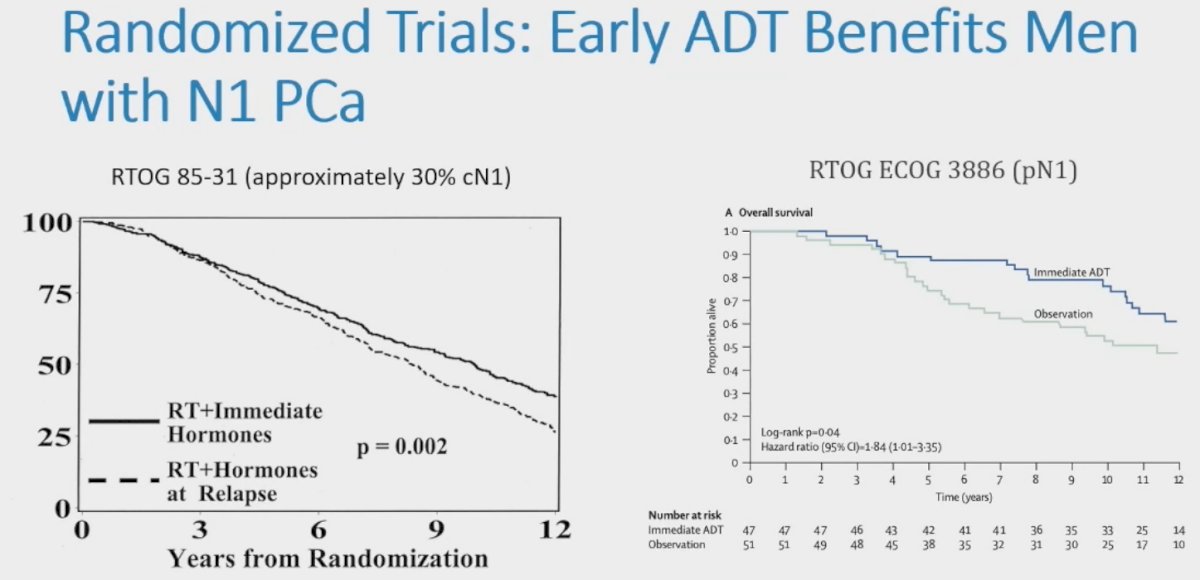
However, to date, there is no data from randomized trials examining the role of local therapy for men with cN1 prostate cancer. As such, is there a benefit to adding local therapy to ADT in men with cN1M0 prostate cancer? 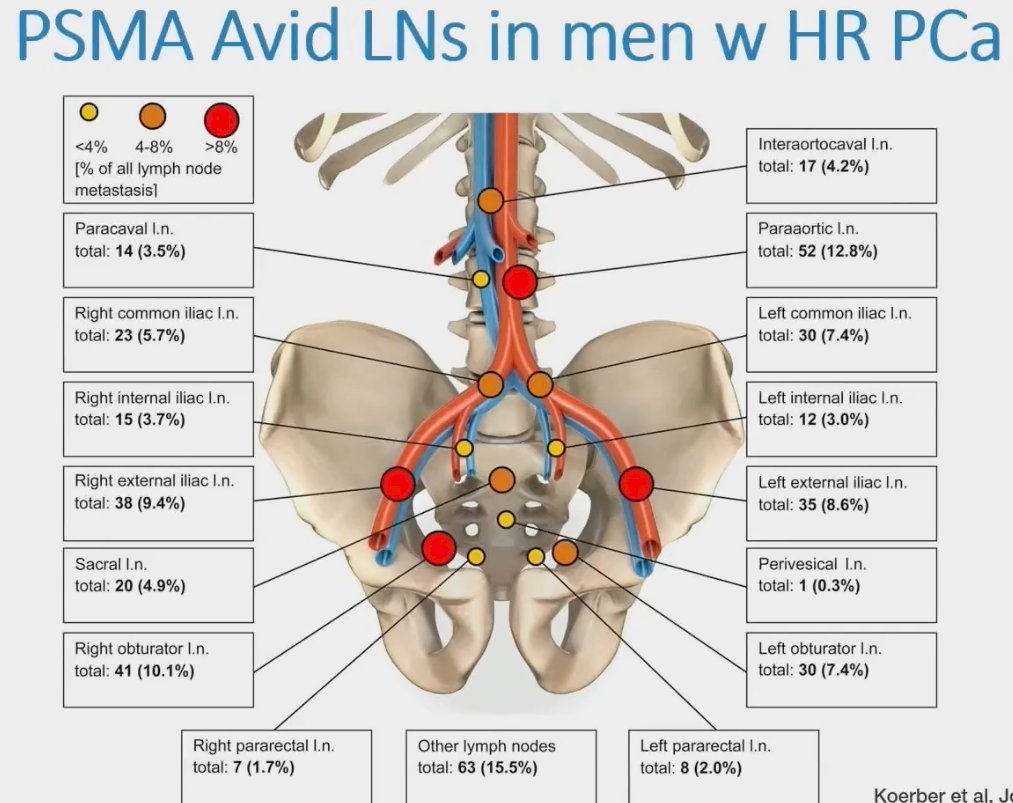
There appears to be a benefit to the addition of radiotherapy for patients with cN1M0 prostate cancer. Non-randomized, ad-hoc analysis of the STAMPEDE control arm evaluated the oncologic benefit of the addition of planned radiotherapy to patients in the standard-of-care hormone therapy arm. This analysis included 177 men with cN1M0 disease. Significantly, failure-free survival outcomes favored the planned use of radiotherapy for patients with both cN0M0 (HR: 0.33, 95% CI: 0.18 – 0.61) and cN+M0 disease (HR: 0.48, 95% CI: 0.29 – 0.79).3
Additional data from the Veterans Affairs database of patients with cN1M0 prostate cancer diagnosed between 2000 and 2015 and treated with ADT (n=450) or ADT + radiotherapy (n=198) demonstrated that definitive treatment with ADT + radiotherapy was associated with improvement in prostate cancer-specific and all-cause mortalities among patients with cN1 prostate cancer and lower baseline PSA levels.
Similarly, a SEER analysis of 1,100 patients with cT1-4N1M0 prostate cancer diagnosed between 1988 and 2006 demonstrated that the 10-year cancer-specific survival was improved for men who had radiation therapy (63% versus 50%). A subsequent National Cancer Database (NCDB) analysis of 3,540 patients with cN1M0 prostate cancer evaluated the benefit of radiotherapy in the clinical node positive setting. In this propensity score-matched analysis of 318 patients per group, it was demonstrated that ADT + radiotherapy was associated with improved:
- 5-year all-cause mortality: HR=0.50, 95% CI: 0.37 – 0.67, p<0.001
- Overall survival: 71.5% versus 53.2%
What about the benefit of ‘intensified’ hormone therapy in the cN+ setting? The STAMPEDE meta-analysis4 demonstrated that the addition of abiraterone 1,000 mg daily + oral prednisolone (5 mg daily) +/- enzalutamide for a median of 20 – 24 months to ADT in 1,974 patients with high-risk disease (defined as node positive [40%], or, if node negative, having at least two of the following: tumour stage T3 or T4, Gleason sum score of 8–10, and PSA concentration ≥40 ng/mL) or high-risk relapse was associated with significant improvements in:
- Metastasis-free survival (HR: 0.53, 95% CI: 0.44 – 0.64)
- Prostate cancer-specific survival (HR: 0.49, 95% CI: 0.37 – 0.65)
- Overall survival (HR: 0.60, 95% CI: 0.48 – 0.73)

The results of this analysis have been reflected in the most recent update of the NCCN guidelines for patients with node-positive disease, where external beam radiotherapy + ADT + abiraterone is the preferred treatment for patients with a >5-year life expectancy. 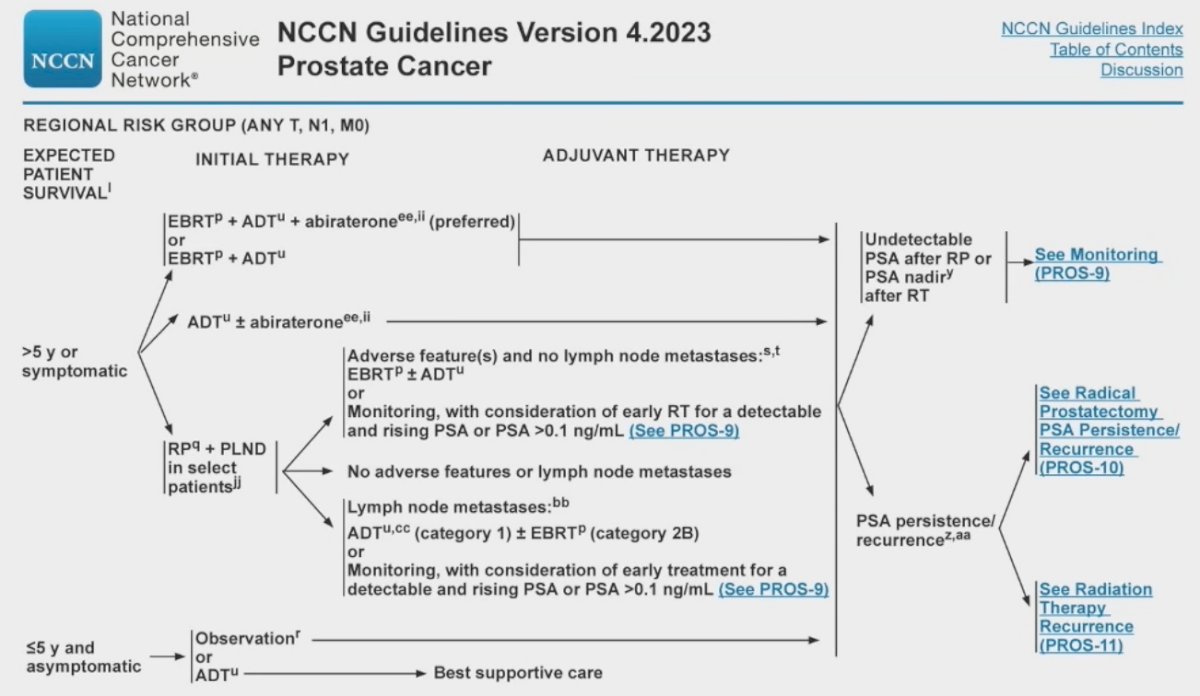
What about patients with cM1a disease involving common iliac and/or para-aortic nodes? Based on data published by Chopade et al. from the MSKCC series, it appears that oncologic outcomes with radiotherapy are similar for patients with cN1 versus M1a disease. This analysis included 130 patients (87 cN1 and 43 M1a), >75% who have been staged with 68Ga PSMA PET/CT and received radiotherapy in the form of 74 Gy to the prostate and seminal vesicles, 45 Gy to the pelvic nodes, and SIB 60 to 66 Gy in 25 fractions to residual nodes >1 cm in size. Significantly, patients with oligometastatic, conventional imaging-defined cM1a and cN1 prostate cancer showed similar outcomes (biochemical failure-free and distant metastasis-free survivals) when treated with curative whole pelvic radiotherapy and long-term ADT.5
Dr. Mian concluded his presentation with the following take home messages:
- The optimal treatment for clinically node positive prostate cancer remains an area of active investigation
- Randomized evidence supports the early addition of intensified hormone therapy
- Retrospective data supports the benefit of local therapy for clinically node positive prostate cancer
- Current evidence-based standard is prostate and pelvic radiotherapy with ADT + abiraterone
- Dr. Mian’s current practice for cN+ patients involves:
- 70 Gy/28F to the prostate and seminal vesicles
- 50.4 Gy/28F to elective pelvis and simultaneous integrated boost to involved nodes to ~60 Gy
Presented by: Omar Mian, MD, PhD, Assistant Professor, Department of Radiation Oncology, Cleveland Clinic, Cleveland, OH
Written By: Rashid K. Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 American Society for Therapeutic Radiation Oncology (ASTRO) 65th Annual Meeting held in San Diego, CA between October 1st and 4th, 2023
References:- Messing EM, Manola J, Yao J, et al. Immediate versus deferred androgen deprivation treatment in patients with node-positive prostate cancer after radical prostatectomy and pelvic lymphadenectomy. Lancet Oncol. 2006;7(6):472-9.
- Pilepich MV, Winter K, Lawton CA, et al. Androgen suppression adjuvant to definitive radiotherapy in prostate carcinoma--long-term results of phase III RTOG 85-31. Int J Radiat Oncol Biol Phys. 2005;61(5):1285=90.
- James ND, Spears MR, Clarke NW, et al. Failure-Free Survival and Radiotherapy in Patients With Newly Diagnosed Nonmetastatic Prostate Cancer: Data From Patients in the Control Arm of the STAMPEDE Trial. JAMA Onc. 2016;2(3):348-57.
- Attard G, Murphy L, Clarke NW, et al. Abiraterone acetate and prednisolone with or without enzalutamide for high-risk non-metastatic prostate cancer: a meta-analysis of primary results from two randomised controlled phase 3 trials of the STAMPEDE platform protocol. Lancet. 2022;399(10323):447-60.
- Chopade P, Maitre P, David S, et al. Common Iliac Node-Positive Prostate Cancer Treated With Curative Radiation Therapy: N1 or M1a? Int J Radiat Oncol Biol Phys. 2022;114(4):711-7.


