(UroToday.com) The 2023 American Society for Radiation Oncology (ASTRO) 65th Annual Meeting held in San Diego, CA between October 1st and 4th, 2023 was host to a session on radiotherapy for kidney cancer and the post-prostatectomy setting. Dr. Paul Nguyen presented FORMULA-509 (Facilitation Of Radiation Management Using Lupron Abiraterone & ARN-509), a multicenter randomized trial of post-operative salvage radiotherapy and 6 months of a GnRH agonist with either bicalutamide or abiraterone acetate and apalutamide.
Dr. Nguyen began by noting that GETUG-16 established 6 months of ADT as a standard adjunct to salvage radiotherapy in the post-prostatectomy setting.1 FORMULA-509 sought to determine whether 6 months of abiraterone acetate plus prednisone and apalutamide would provide superior outcomes to bicalutamide for patients with higher risk features receiving salvage radiation and 6 months of a GnRH agonist. This trial included patients with a PSA >0.1 ng/ml following radical prostatectomy and at least 1 unfavorable risk factor:
- pT3 disease
- Gleason Score 8-10
- PSA >0.5 ng/ml
- Pathologically positive lymph nodes
- PSA doubling time <10 months
- Negative margins
- Persistent PSA after surgery
- Local recurrence on imaging
- Decipher ‘high risk’
Of note, patients with bone metastasis or nodal disease outside the pelvis were excluded. The trial design is summarized below. In brief, patients underwent stratified randomization (by PSA >0.5 versus ≤0.5 ng/ml and pN1 versus pN0) to either:
- Salvage XRT + 6 months of GnRH agonist + bicalutamide (active comparator)
- Salvage XRT + 6 months of GnRH agonist + abiraterone acetate/prednisone + apalutamide
The primary outcome was progression-free survival (PFS), with secondary outcomes of metastasis-free survival (MFS) and both physician and patient-reported toxicities. The study had 80% power to detect a PFS HR of 0.50 and an MFS HR of 0.30, with a one-sided alpha of 0.05 for each. There were pre-planned subgroup analyses by the two stratification factors (PSA >0.5 and pN1) using two-sided p-values.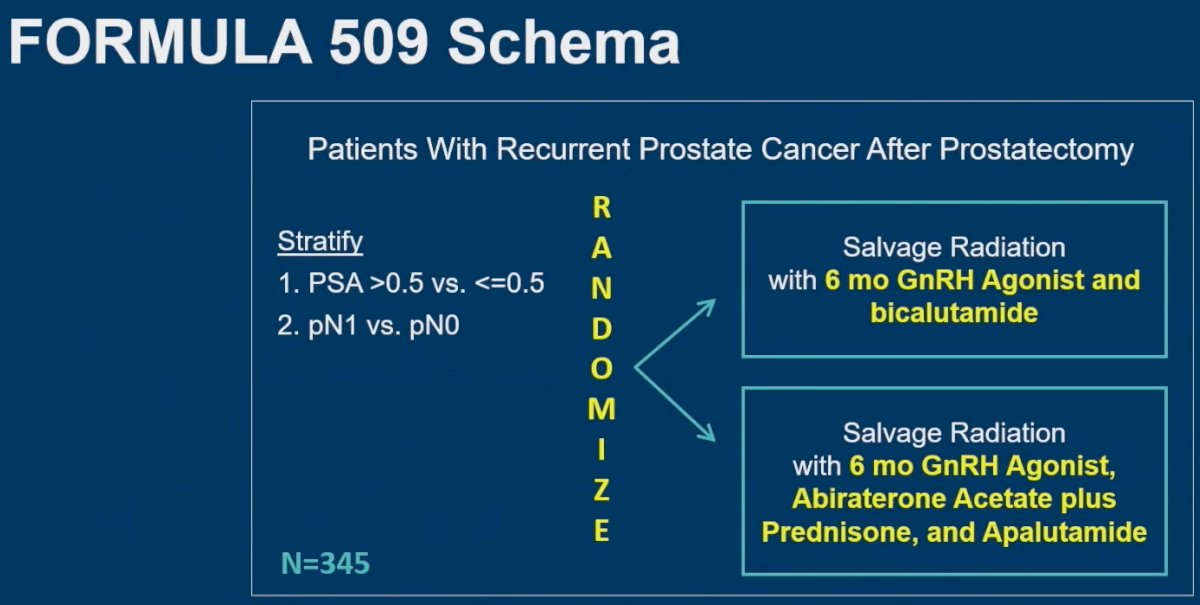
The baseline patient characteristics of the 345 trial patients are summarized below. The median patient age was 65 years. 35% of patients had Gleason score 9 disease, the median PSA was 0.3 ng/ml, consistent with contemporary practice (31% had PSA >0.5 ng/ml), and 29% had pN1 disease.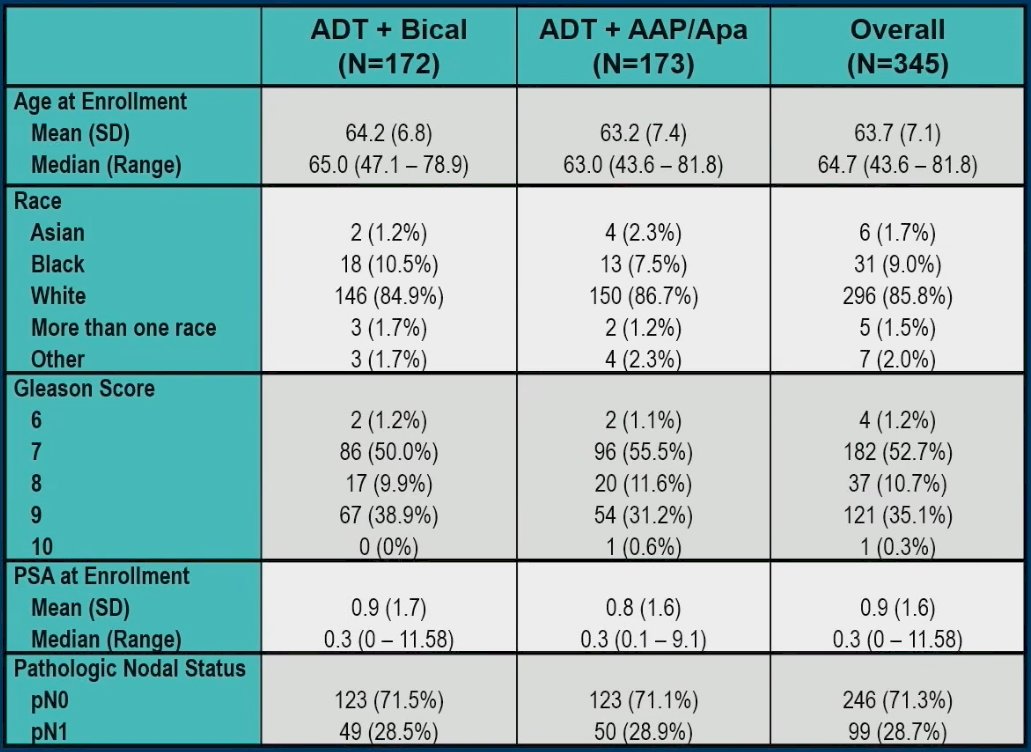
The median radiation dose was a standard dose of 68.4 Gy and 74% of patients received pelvic nodal radiation.
At a median follow-up of 34 months, there was no significant improvement in 3-year PFS (75% versus 68.5%; HR= 0.71, 90% CI: 0.49 – 1.03, p=0.06).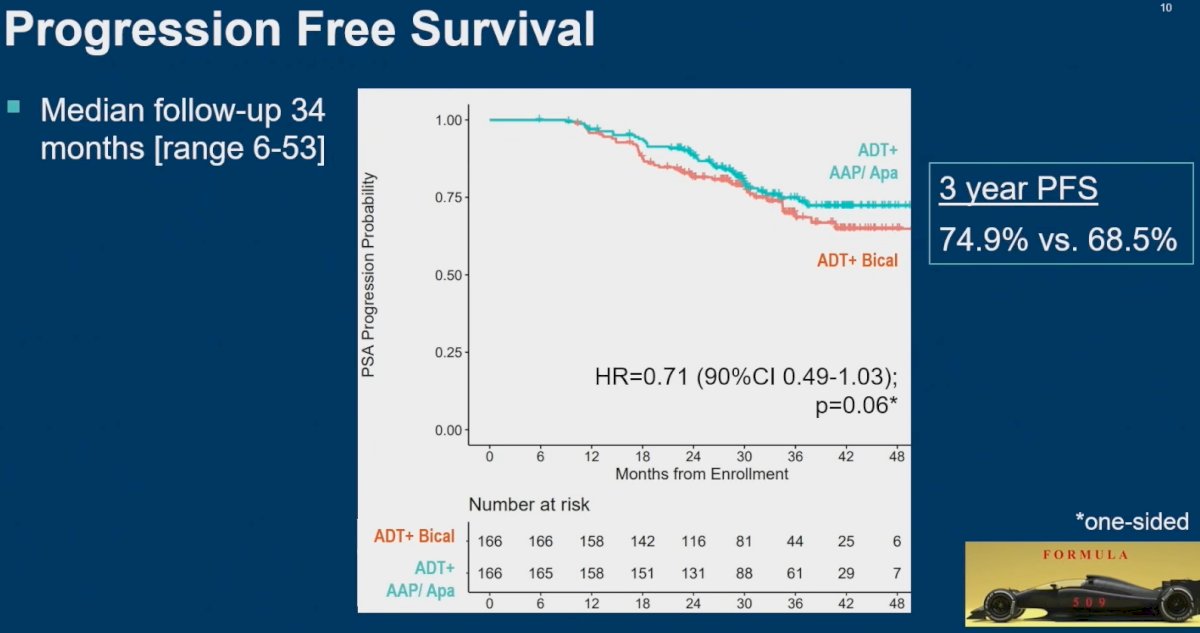
Similarly, there was no significant improvement in MFS, with 3-year MFS of 91% and 87% in the experimental and control arms, respectively (HR: 0.57, 90% CI: 0.33 – 1.01, p=0.05).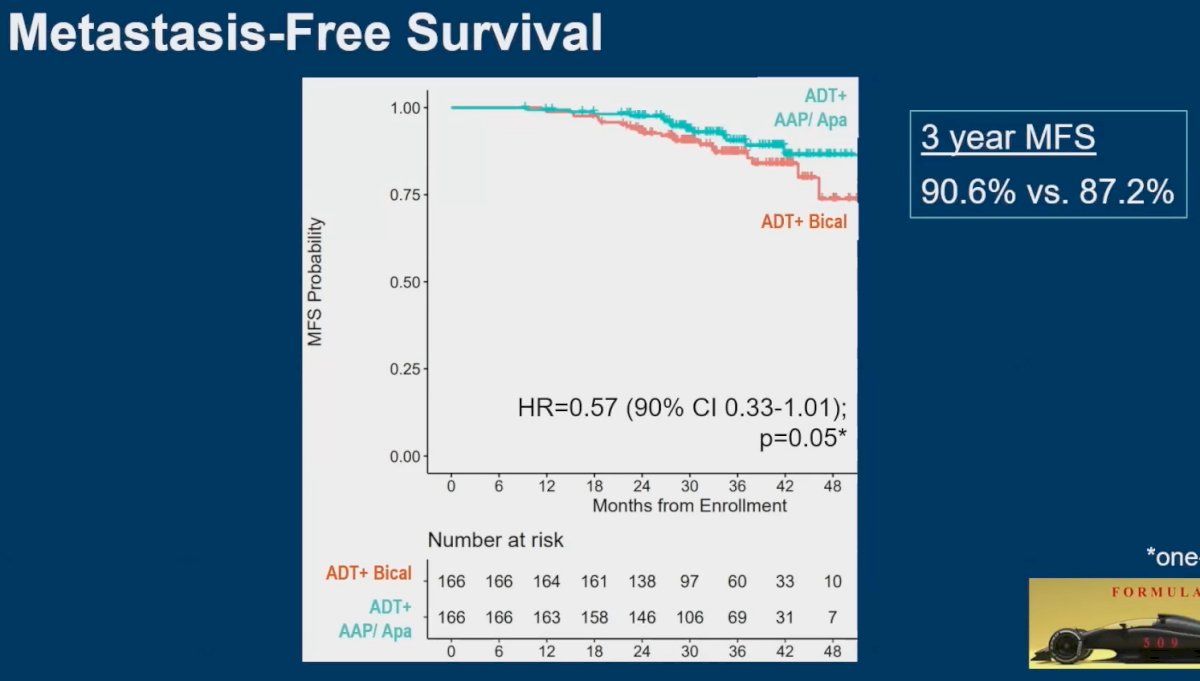
What about the subgroup analysis among patients with a PSA >0.5 ng/ml (n=100)? It appears that the addition of abiraterone and apalutamide to a GnRH agonist in the post-prostatectomy salvage setting significantly improves PFS outcomes (3-year PFS: 67% versus 47%; HR: 0.50, 95% CI: 0.27 – 0.95. p=0.03).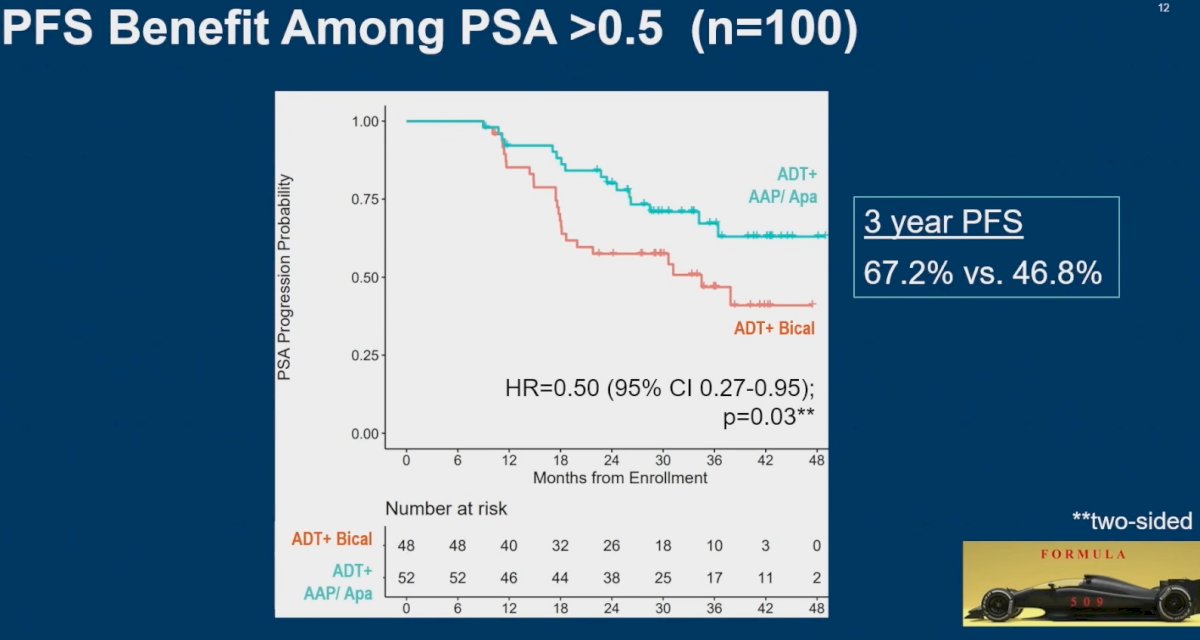
Similarly, an MFS benefit was observed with the combination treatment in patients with a PSA >0.5 ng/ml, with improvement in 3-year MFS from 66% to 84% (HR: 0.32, 95% CI: 0.13 – 0.84, p=0.02). Dr. Nguyen again emphasized that these subgroup analyses were pre-planned at the time of trial design.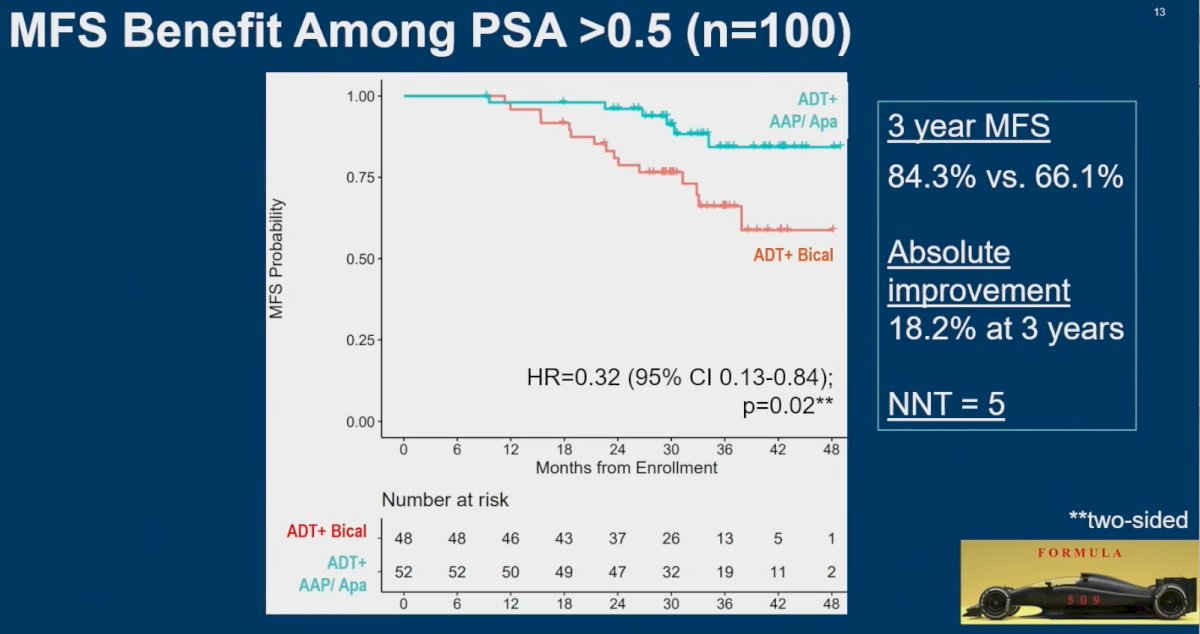
The results of the subgroup analyses are summarized in the forest plot below: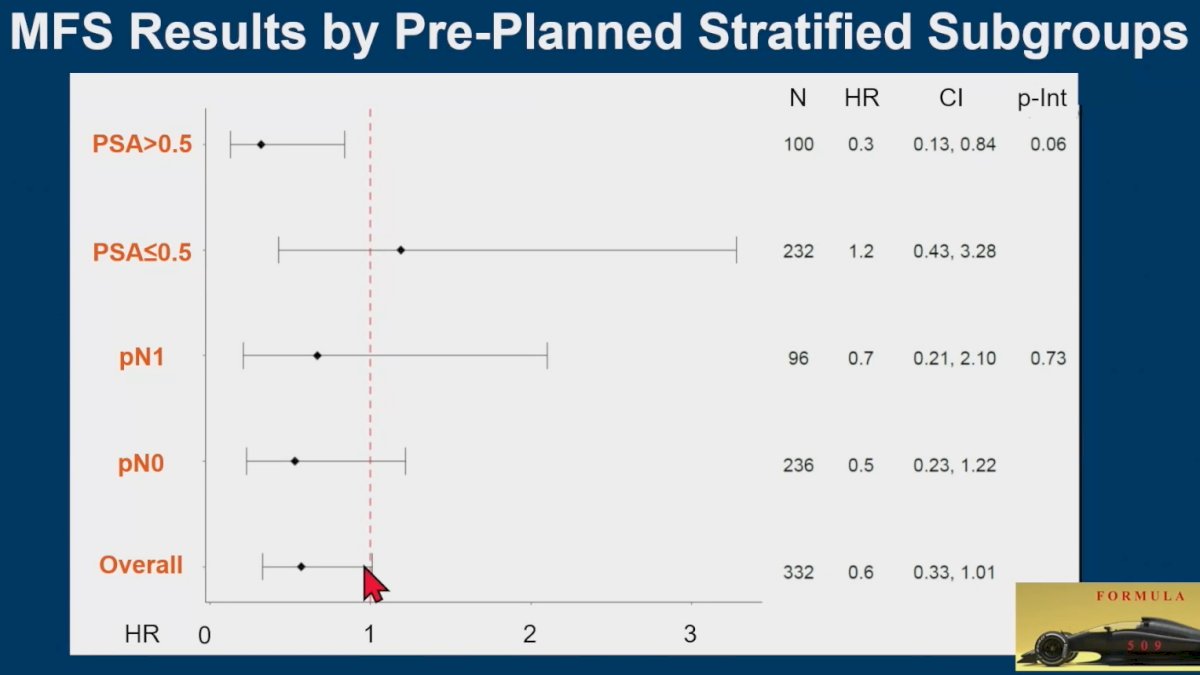
The adverse events were consistent with the known safety profiles of abiraterone and apalutamide. There was an increased incidence of grade 2+ maculopapular rash, hypertension, and diarrhea in the ADT + abiraterone/apalutamide arm.
How do we contextualize the results of this trial in the setting of the previously published RADICALS-HD trial? One important difference is that the experimental arm in the RADICALS-HD trial received 24 months of ADT, compared to the shorter, but more ‘intense’ 6 months in the FORMULA-509 trial. While cross-trial comparisons are fraught with inherent biases, comparison of the MFS outcomes, as summarized below, demonstrates that the MFS magnitude of benefit favors the FORMULA-509 trial (HR of 0.57 versus 0.77). This is similarly noted in the PSA >0.5 ng/ml subgroup, with a HR of 0.32 (versus 0.67) in favor of the FORMULA-509 trial. As such, Dr. Nguyen argued that for patients with higher risk features, intensifying 6 months of ADT may be an appealing alternative to lengthening ADT duration to 24 months.
Dr. Nguyen concluded his presentation as follows:
- Although the primary analysis of FORMULA-509 with 34 months follow-up did not meet the pre-specified threshold for statistical significance, it does strongly suggest that the addition of abiraterone acetate + apalutamide to salvage radiotherapy + 6 months of ADT may improves PFS and MFS, particularly in the subgroup of patients with PSA >0.5 ng/ml, where a pre-planned subgroup analysis by stratification factors observed a statistically significant benefit in PFS and MFS.
- 6 months of intensified ADT with next generation anti-androgens may provide an attractive alternative to lengthening ADT for patients with rising PSA and unfavorable features after radical prostatectomy.
Presented by: Paul Nguyen, MD, Professor, Department of Radiation Oncology, Dana-Farber/Brigham and Women’s Hospital, Boston, MA
Written By: Rashid K. Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 American Society for Therapeutic Radiation Oncology (ASTRO) 65th Annual Meeting held in San Diego, CA between October 1st and 4th, 2023
References:

