(UroToday.com) The 2023 American Society for Radiation Oncology (ASTRO) 65th Annual Meeting held in San Diego, CA between October 1st and 4th, 2023 was host to a session on radiotherapy for kidney cancer and the post-prostatectomy setting. Dr. Alan Dal Pra discussed the results of a secondary analysis of the phase 3 NRG/RTOG 0534 SPPORT trial evaluating the impact of testosterone recovery on clinical outcomes of patients treated with salvage radiotherapy and androgen suppression.
Dr. Dal Pra began by noting that post-treatment serum testosterone kinetics and its relationship with clinical outcomes has not been studied in trials of salvage radiotherapy plus ADT. As such, the authors sought to investigate longitudinal serum testosterone levels and the impact of testosterone recovery as a secondary analysis of the NRG Oncology/RTOG 0534 SPPORT trial,1 which compared:
- Arm 1: prostate bed radiotherapy (PBRT)
- Arm 2: PBRT + short-term ADT
- Arm 3: PBRT + short-term ADT + pelvic nodal radiotherapy
Patients in Arms 2 and 3 received ADT for 4-6 months, starting 2 months prior to salvage radiotherapy. Patients with baseline testosterone levels <40% of the lower limit of normal were excluded. In this study, testosterone recovery was defined in 3 ways:
- Return to non-castrate level (>50 ng/dL)
- Return to normal level (>300 ng/dL)
- Return to baseline level
The following study outcomes were assessed:
- Time to testosterone recovery
- Time to distant metastasis
- Cause-specific mortality
- Time to initiation of second salvage ADT
- These 4 outcomes were assessed using cumulative incidence curves with other-cause death treated as a competing risk
- Freedom from progression
- Defined as biochemical failure (Phoenix definition), clinical failure, or death from any cause
- Overall survival
- Estimated using Kaplan-Meier method
For the time-to-event outcomes, unadjusted and adjusted hazard ratios, along with the 95% confidence intervals were calculated using Cox proportional hazards modeling.
The baseline patient characteristics are summarized below. Of note, the mean patient age was 64 years, 13% were Black, 93% had an excellent performance status, and 15% had diabetes.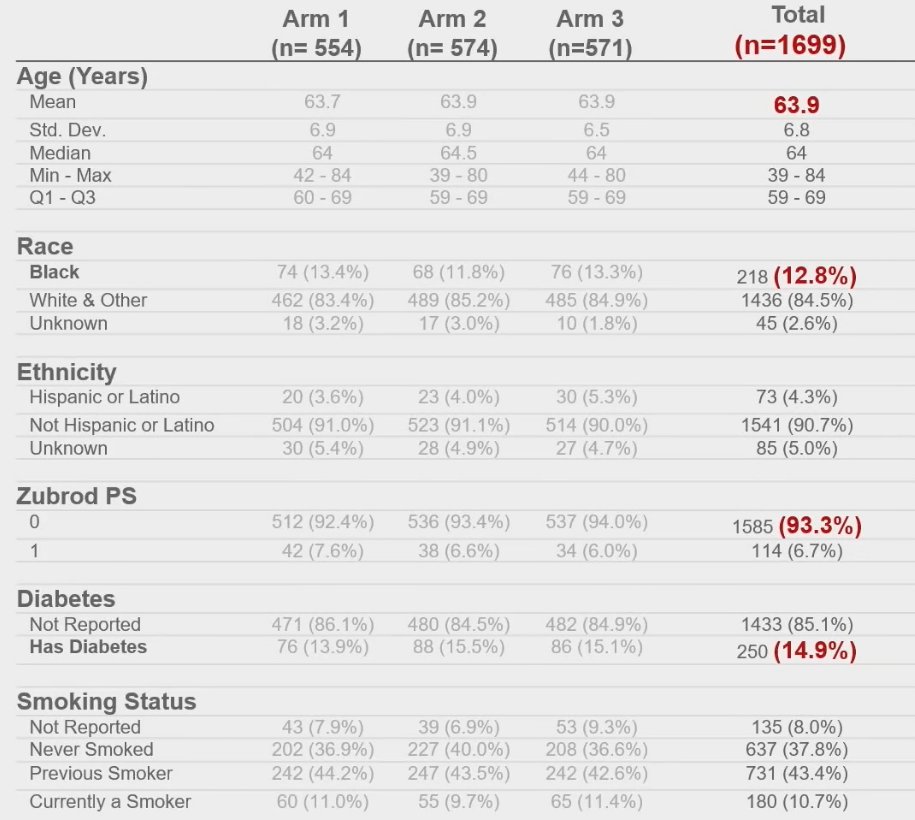
Longitudinal analysis of testosterone levels by treatment arm demonstrated that median testosterone levels decreased to castrate levels by week 6 in patients from Arms 2 and 3. By one year, the median testosterone level was equivalent in all 3 arms. Interestingly, the median testosterone level decreased by approximately 15% by 12 months in Arm 1 that received PBRT only.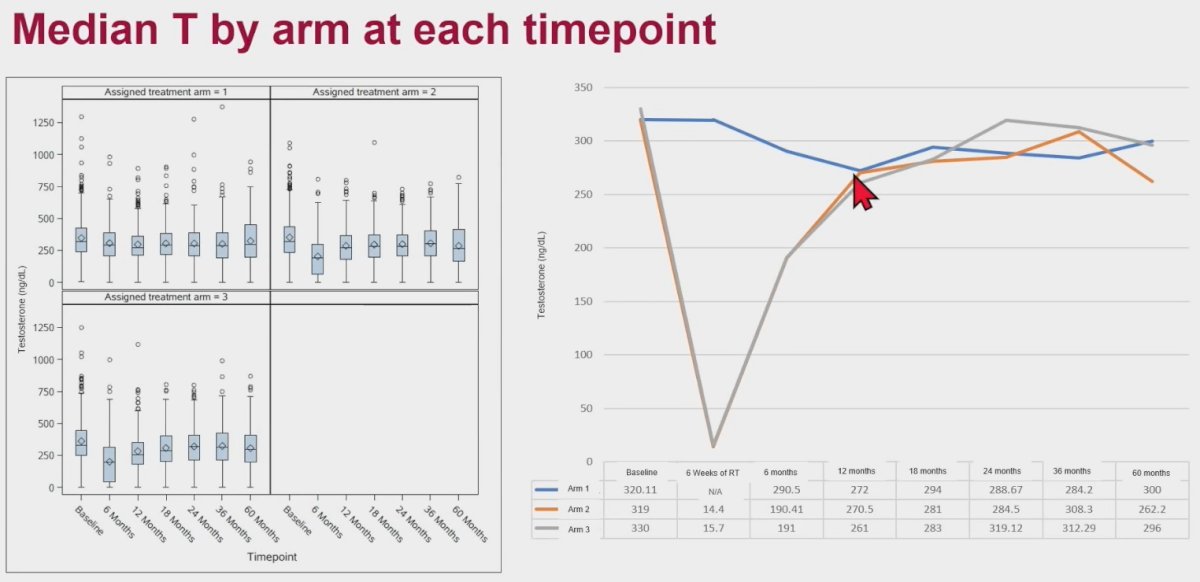
As demonstrated in the table below, by 24 months, testosterone recovery among patients in Arms 2 and 3 were as follows:
- Non-castrate level: 95%
- Normal levels: 54 – 56%
- Baseline levels: 23%

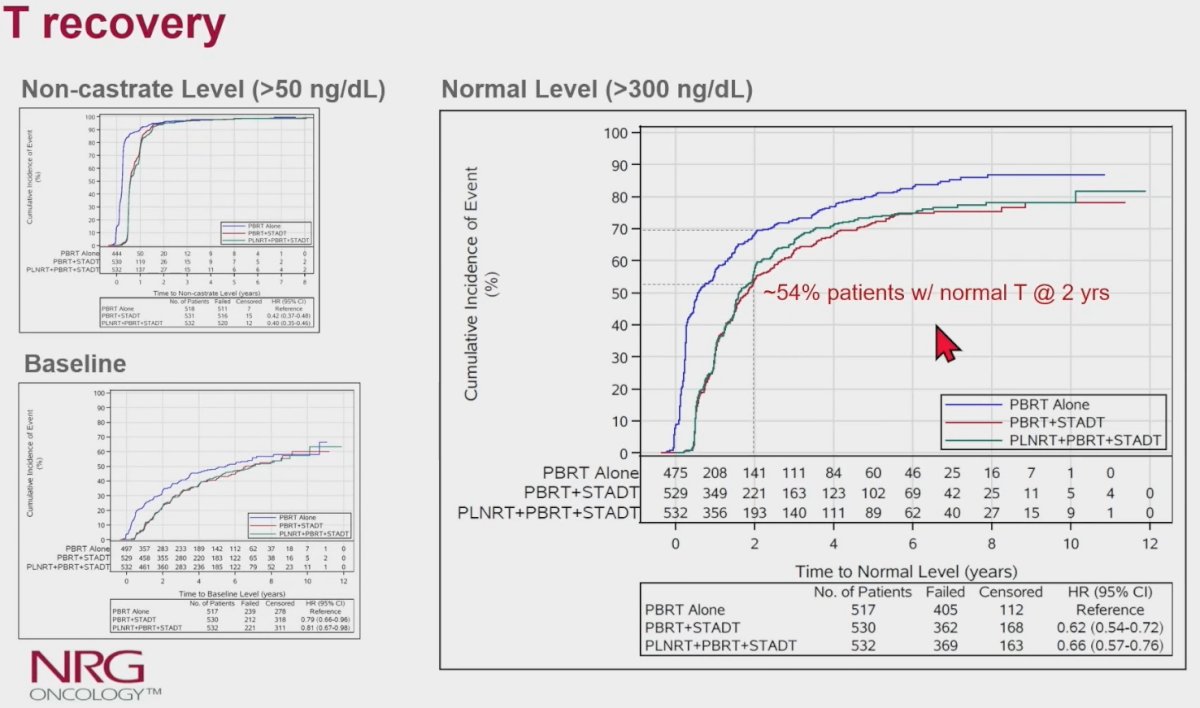
The median time to testosterone recovery to normal level was 19 – 21 months from study enrolment (i.e., 13 – 15 months from the end of the last injection).
Does testosterone recovery impact clinical outcomes? With regards to the outcome of freedom-from-progression, this outcome was superior in patients with recovered testosterone levels in Arms 2 and 3 versus Arm 1, irrespective of the definition of testosterone recovery used.
Similarly, among patients with recovered testosterone levels, the initiation of second salvage ADT was higher among patients in Arm 1, compared to those in arms 2 or 3. There were no differences in distant metastasis, cancer-specific mortality, or overall survival outcomes.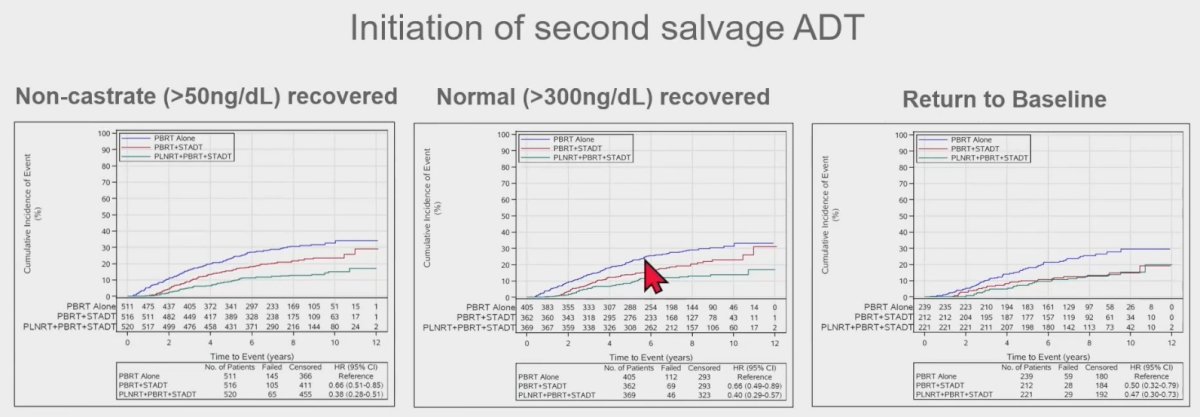
What about the subgroup of patients who recover testosterone within 2 years? Similarly, freedom-from-progression was superior among those in Arms 2 and 3, compared to Arm 1.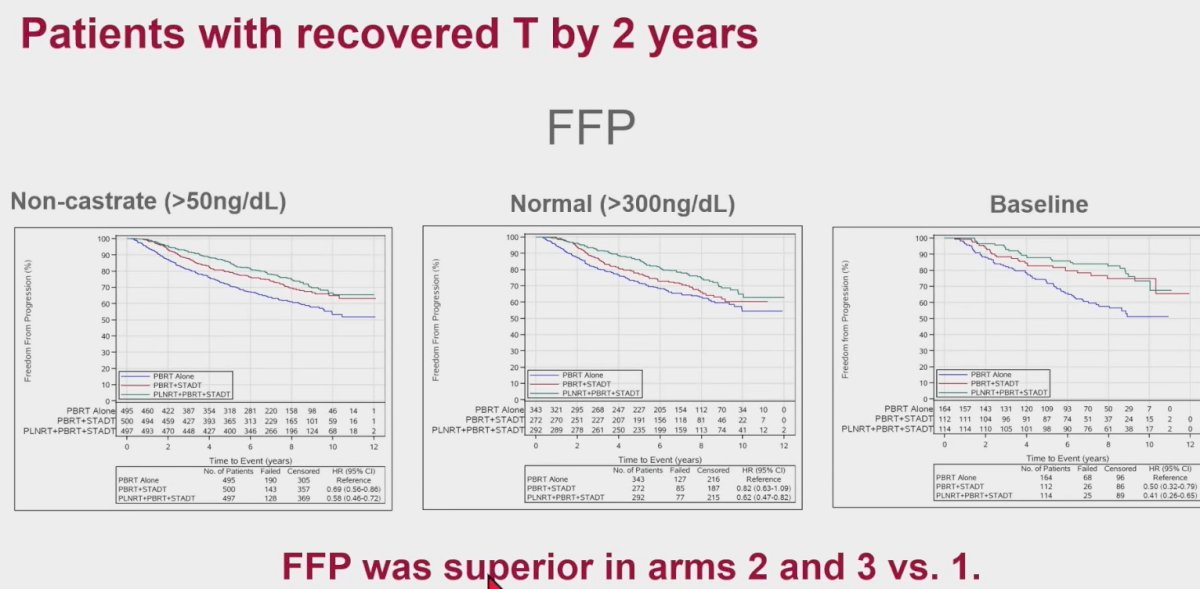
When these results are analyzed in the context of the trial results published by Pollack et al. in 2022, we see no significant differences in the primary outcome of freedom from progression between those in the full cohort and those with recovered testosterone level (>300 ng/dL) by 2 years, reassuring us that outcomes do not worsen among those who recover their testosterone levels.
Based on these results, Dr. Dal Pra discussed that among well-selected patients with good performance status and likely higher serum testosterone levels compared to the general prostate cancer population (those <40% of the lower limit of normal were excluded):
- Normalization of serum testosterone levels does not affect the outcomes of patients treated with PBRT + short-term ADT + pelvic nodal radiotherapy, consistent with the saturation model theory
- These results have potential clinical implications for the use of oral GnRH antagonists that have faster testosterone recovery and testosterone replacement therapy in prostate cancer patients with further studies warranted in this setting
In conclusion:
- This study represents the largest analysis of testosterone kinetics in patients treated with salvage radiotherapy and ADT
- Approximately half of patients receiving short-term ADT did not normalize testosterone levels by 2 years
- This data support NRG/RTOG 0534 SPPORT results of an incremental freedom-from-progression benefit of adding short-term ADT and pelvic nodal radiotherapy independent of testosterone recovery
Presented by: Alan Dal Pra, MD, Associate Professor, Department of Radiation Oncology, University of Miami, Miami, FL
Written By: Rashid K. Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 American Society for Therapeutic Radiation Oncology (ASTRO) 65th Annual Meeting held in San Diego, CA between October 1st and 4th, 2023
References:

