(UroToday.com) The 2023 American Society for Radiation Oncology (ASTRO) 65th Annual Meeting held in San Diego, CA between October 1st and 4th, 2023 was host to a session discussing combined modality therapy for lymph node positive prostate cancer. Dr. Hala Borno discussed the current state of modern systemic therapy for advanced prostate cancer.
Dr. Borno began her presentation with a case study of a 75-year-old male with a PSA of 8 ng/ml and, slightly enlarged prostate on a rectal exam, with a subsequent prostate biopsy demonstrating Grade Group 2 disease in 6/12 cores. Staging work up with conventional imaging (CT and bone scan) was negative for metastatic disease. Labs were otherwise unremarkable. He underwent a radical retropubic prostatectomy with final pathology demonstrating:
- Gleason score 8 disease involving 60% of the gland, with capsular penetration and right seminal vesicle involvement
- 1 of 5 lymph nodes on the right side were positive for metastatic disease
In the absence of additional diagnostics, what is the best treatment option?
- Observation followed by deferred ADT
- Immediate ADT monotherapy
- Immediate ADT combined with abiraterone acetate and prednisone
- Immediate ADT combined with abiraterone acetate and enzalutamide
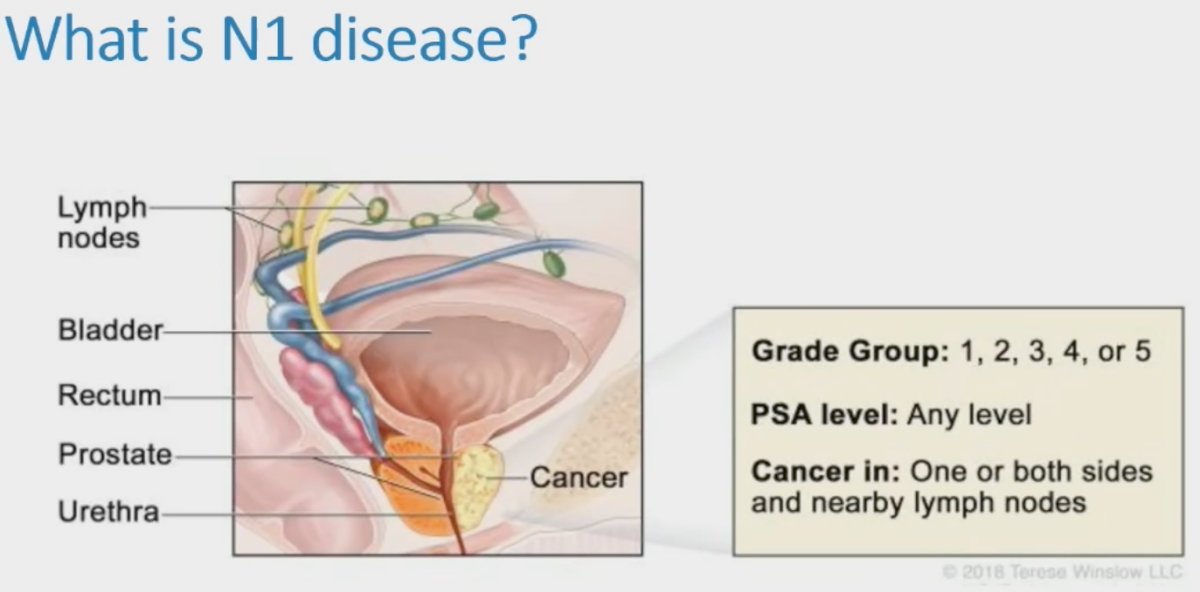
The incidence of N1 disease appears to be increasing, with a recent report of SEER data (2004 to 2014) demonstrating that the age-standardized incidence of N1 disease has been increasing over the last decade, and this has a clear adverse impact on mortality outcomes with cN+ patients having a 4.5-fold increased rate of prostate cancer related deaths.1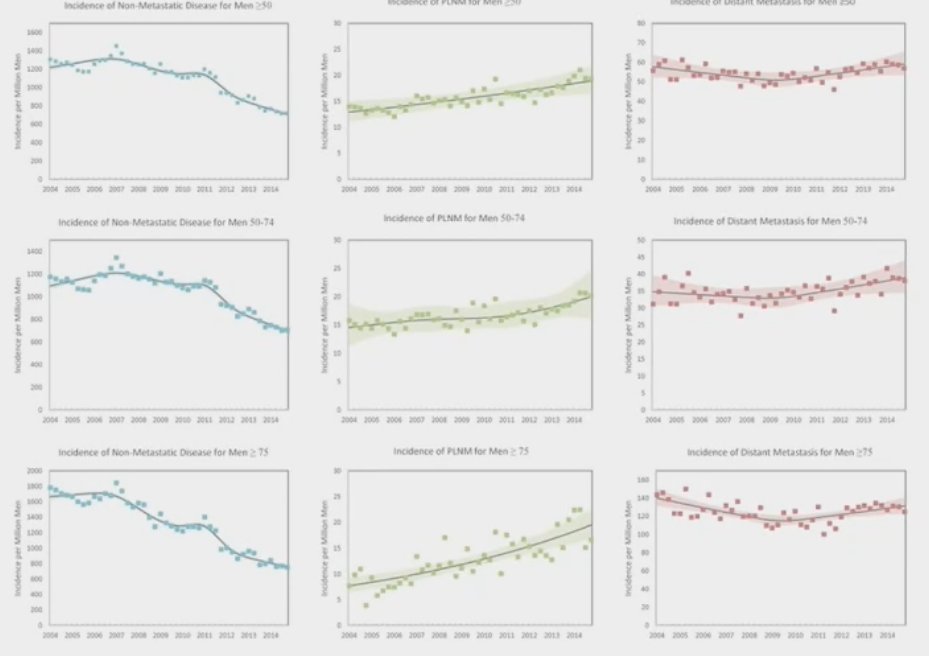
With the emergence of advanced molecular imaging with PSMA PET over the last few years, we have seen a shift in the clinical staging of higher risk patients. What is the current role of conventional imaging for nodal staging? Currently, there remain strong guideline recommendations to perform metastatic screening, including at least one cross-sectional abdominopelvic imaging and a bone scan, with current size cut-offs for pelvic cN1 disease defined as 8 mm in the short axis or 10 mm for oval lymph nodes. However, it is quite clear that conventional imaging performs poorly for staging pelvic cN1 disease with sensitivities of 11-13% and 39-56% for CT and MRI, respectively.
Advanced molecular imaging clearly outperforms conventional imaging for the detection of nodal disease. ProPSMA was a multi-center, two-arm randomized controlled trial of men with histologically confirmed prostate cancer who were being considered for curative intent radical prostatectomy or radiotherapy. To be eligible for inclusion, men must have had at least one high-risk factor including PSA ≥ 20 ng/mL, ISUP grade group 3-5, or clinical stage T3 or greater. Following enrollment, patients were randomly assigned in a 1:1 ratio to either conventional imaging consisting of bone scan and CT or 68Ga-PSMA-11 PET/CT. Confirmatory testing following imaging was performed at the discretion of the treating physician and included biopsy confirmation.
Between 2017 and 2018, 302 patients were randomized to either conventional imaging (n = 152) or 68Ga-PSMA-11 PET/CT (n = 150). With regards to the primary outcome, PSMA PET/CT had a 27% absolute greater AUC for accuracy compared to conventional imaging (95% CI for difference: 23 – 31%): 92% (95% CI: 88 – 95%) vs. 65% (95% CI: 60 – 69%). Conventional imaging had both a lower sensitivity (38% vs. 85%) and specificity (91% vs. 98%). Subgroup analyses by site of metastasis demonstrated the superiority of PSMA PET/CT for pelvic nodal (AUC: 91% versus 59%) and distant metastases (AUC: 95% versus 74%).2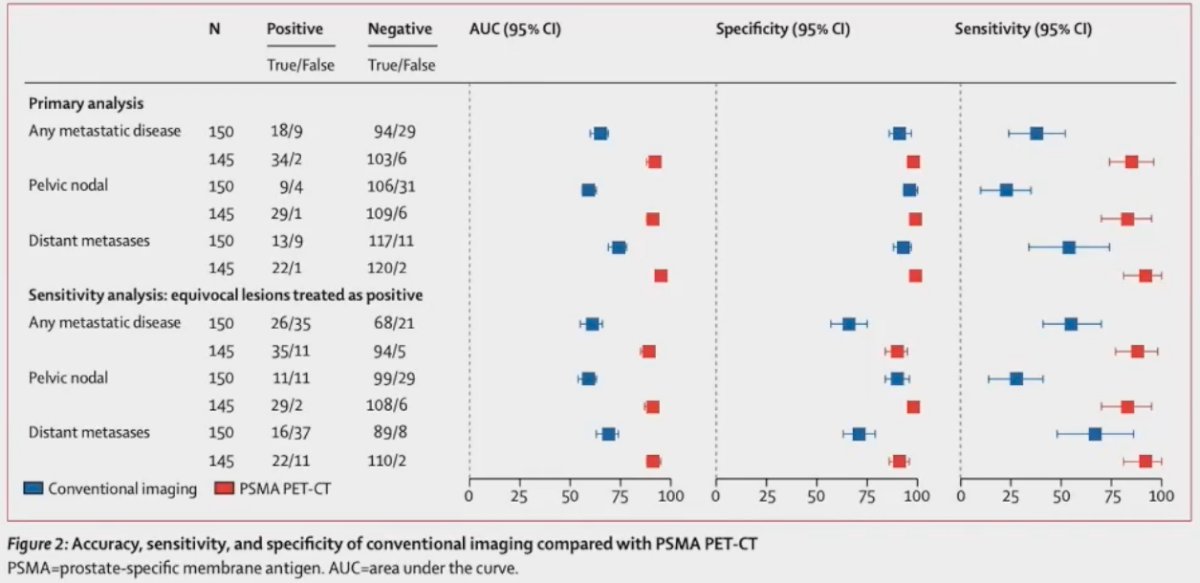
However, there are important limitations for nodal staging with PSMA PET. A recently published meta-analysis by Stabile et al. demonstrated that the performance of PSMA PET for the staging of nodal disease in the pre-operative setting, namely the positive (PPV) and negative predictive values (NPV), are highly dependent on the pre-operative nodal involvement risk. As demonstrated below, the PPV significantly increases as the prevalence of LN involvement increases, with the reverse seen for the NPV.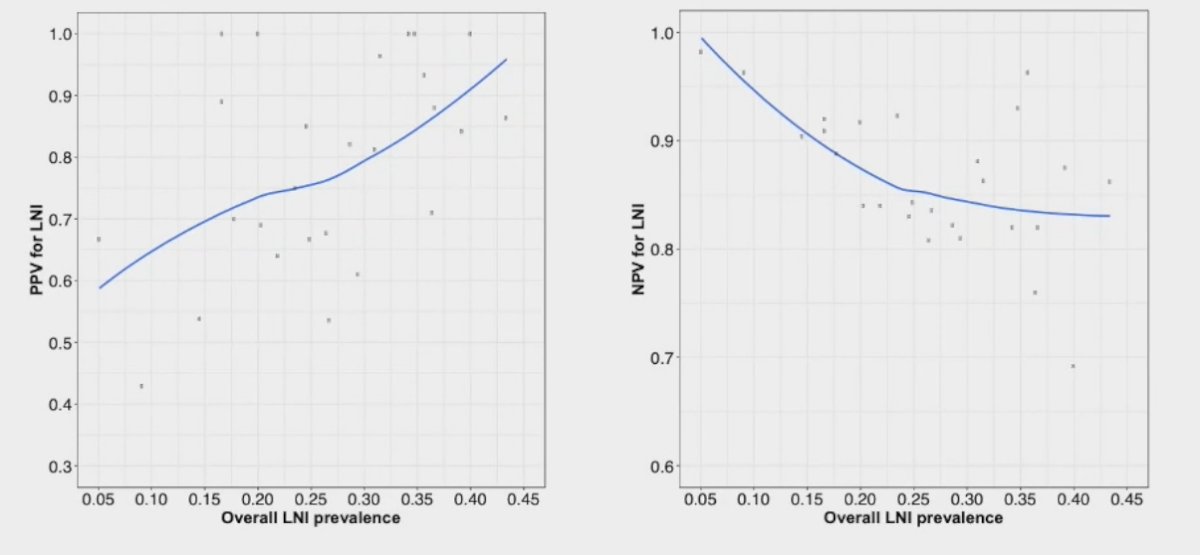
Next, Dr. Borno moved on to discuss the management of node-positive disease. Current guidelines consistently recommend the use of ADT in N+ patients, irrespective of whether this clinical or pathologic, with the option of adding abiraterone to ADT in patients with cN+ disease on the basis of the STAMPEDE meta-analysis demonstrating a clinical benefit in this setting.
The only randomized data to support the use of early ADT in patients with evidence of pathologic nodal disease originates from the ECOG 3886 trial, published by Messing et al. in 2006.3 This trial randomized 98 patients to immediate versus delayed (at time of distant metastases or symptomatic recurrences) lifelong ADT in pN+ patients. The median number of involved nodes was 2. Immediate ADT was associated with significant improvements in:
- Overall survival: HR=1.84
- Cancer specific survival: HR= 4.09
- Progression-free survival: HR= 3.42
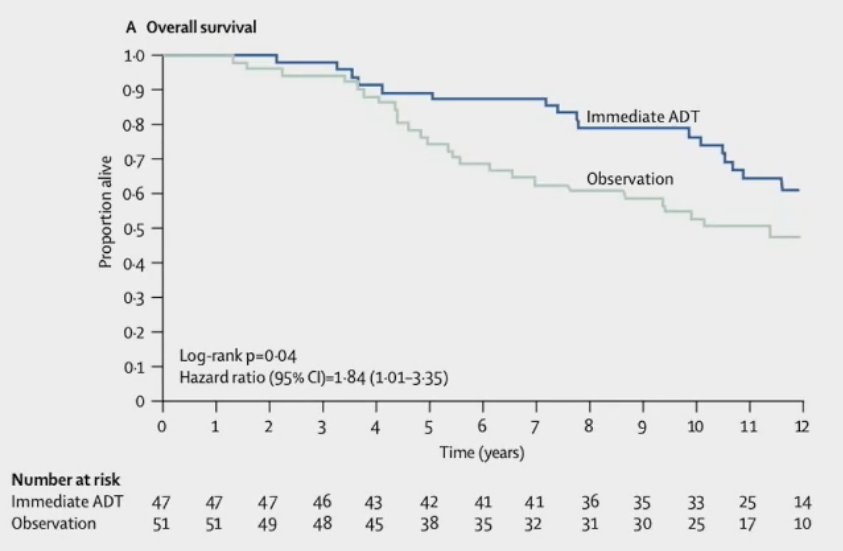
As such, based on this data, it appears that observation followed by deferred ADT is not the ideal treatment choice for patients with pN+ disease following surgery. What about the role of additional systemic therapy concurrently with ADT?
In 2016 Vale et al. published a systematic review of GETUG-12, RTOG 0521, and STAMPEDE that evaluated the role of docetaxel addition to ADT in patients with non-metastatic disease. The addition of docetaxel in this setting was not associated with improvements in metastasis-free or overall survival.4
The evidence for abiraterone addition to ADT in cN+ patients originates from the STAMPEDE meta-analysis5 that demonstrated that the addition of abiraterone 1,000 mg daily + oral prednisolone (5 mg daily) +/- enzalutamide for a median of 20 – 24 months to ADT in 1,974 patients with high-risk disease (node positive: 40%) or high-risk relapse was associated with significant improvements in:
- Metastasis-free survival (HR: 0.53, 95% CI: 0.44 – 0.64)
- Prostate cancer-specific survival (HR: 0.49, 95% CI: 0.37 – 0.65)
- Overall survival (HR: 0.60, 95% CI: 0.48 – 0.73)

What is the role of triplet therapy in the cN+ setting? To date, there is no evidence to support this practice, as there is no prospective randomized controlled trial to demonstrate a significant benefit for triplet therapy. Formula509 in the biochemical recurrent setting suggests that the addition of abiraterone acetate/prednisone + apalutamide to salvage radiotherapy and 6 months of ADT improves progression-free and metastasis-free survivals; however, the pN1 subgroup did not benefit. Similarly, the STAMPEDE meta-analysis demonstrated that the addition of enzalutamide to abiraterone did not improve outcomes for patients with high-risk prostate cancer, including cN+ patients.5
Based on the current body of evidence, Dr. Borno concluded that it appears that the best systemic treatment option for patients with cN+ disease is immediate ADT combined with abiraterone acetate and prednisone. However, many questions remain in applying a risk-adapted strategy:
- How do we take into account the number of lymph node metastases as data has shown that limited lymph node metastases tend to have better outcomes?
- How do we factor in pathologic and clinical characteristics?
- How do we factor in N1 disease detected on molecular versus conventional imaging?
- What is the role of surgical margin and T staging for node positive disease?
- How do we incorporate predictive rather than prognostic biomarkers?
Presented by: Hala Borno, MD, Associate Professor, Department of Medicine, Division of Hematology Oncology, University of California, San Francisco, CA
Written By: Rashid K. Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 American Society for Therapeutic Radiation Oncology (ASTRO) 65th Annual Meeting held in San Diego, CA between October 1st and 4th, 2023
References:- Bernstein AN, Shoag JE, Golan R, et al. Contemporary Incidence and Outcomes of Prostate Cancer Lymph Node Metastases. J Urol. 2018;199(6):1510-7.
- Hofman MS, Lawrentschuk N, Francis RJ, et al. Prostate-specific membrane antigen PET-CT in patients with high-risk prostate cancer before curative-intent surgery or radiotherapy (proPSMA): a prospective, randomised, multicentre study. Lancet. 2020;395(10231):1208-16.
- Messing EM, Manola J, Yao J, et al. Immediate versus deferred androgen deprivation treatment in patients with node-positive prostate cancer after radical prostatectomy and pelvic lymphadenectomy. Lancet Oncol. 2006;7(6):472-9.
- Vale CL, Burdett S, Rydzewska LH, et al. Addition of docetaxel or bisphosphonates to standard of care in men with localised or metastatic, hormone-sensitive prostate cancer: a systematic review and meta-analyses of aggregate data. Lancet Oncol. 2016;17(2):243-56.
- Attard G, Murphy L, Clarke NW, et al. Abiraterone acetate and prednisolone with or without enzalutamide for high-risk non-metastatic prostate cancer: a meta-analysis of primary results from two randomised controlled phase 3 trials of the STAMPEDE platform protocol. Lancet. 2022;399(10323):447-60.


