(UroToday.com) The 2023 ASTRO annual meeting included a session on exploring the ethical and legal implications of artificial intelligence in medical practice, featuring a presentation by Dr. Sushil Beriwal discussing artificial intelligence tools in radiation oncology clinical practice. Dr. Beriwal started by highlighting that artificial intelligence is the simulation of human intelligence processes by machines, especially computer systems, and the ability of computer algorithms to approximate conclusions based solely on input data. These systems work by:
- Ingesting large amounts of labeled training data
- Analyzing the data for correlations and patterns
- Using these patterns to make predictions about future states
There are several goals for artificial intelligence in radiation therapy:
- Improve efficiency and save time
- Uniformity of care
- Improve quality of care
- Improve access to care
- Help with training
Several examples of artificial intelligence in the radiation therapy process include (i) image acquisition, (ii) segmentation, (iii) physician plan of care, (iv) treatment planning, (v) quality assurance, (vi) treatment delivery, and (vii) outcome prediction.
With regards to image acquisition and enhancement, Dr. Beriwal notes that artificial intelligence may help replace CT in MR based treatment planning and facilitate CBCT-based image-guided adaptive radiotherapy. This may have particular importance in brachytherapy where images are often acquired under certain practical constraints (ie. software or hardware availability and applicator compatibility). As follows is an MRI only workflow with synthetic CT:

An example for prostate cancer, using MRI only workflow with synthetic CT is follows:
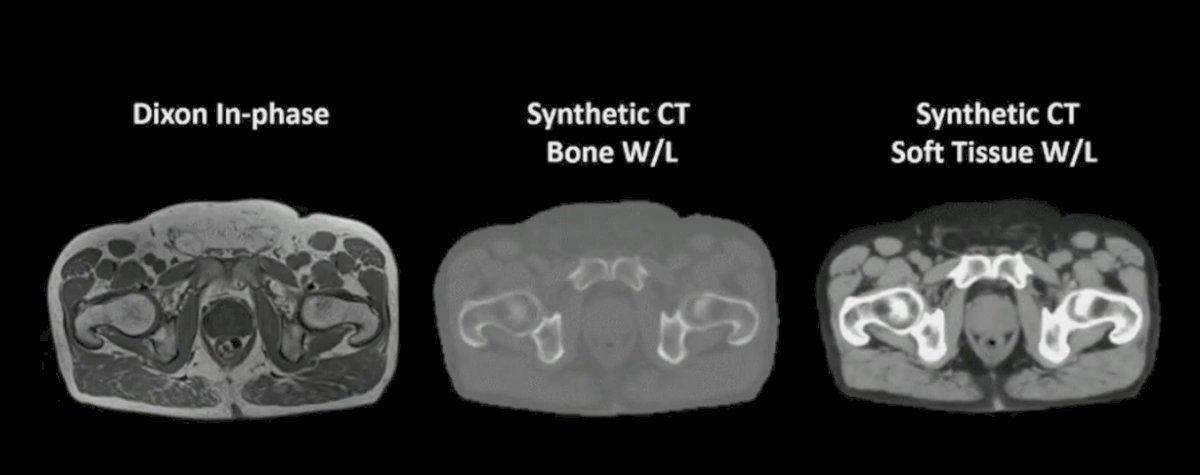
Additionally, artificial intelligence can assist with decreasing exposure and producing higher quality, enhanced images without interruption from artifact.
For segmentation and artificial intelligence, Dr. Beriwal emphasized that it is important to understand the metrics for evaluating artificial intelligence segmentation:
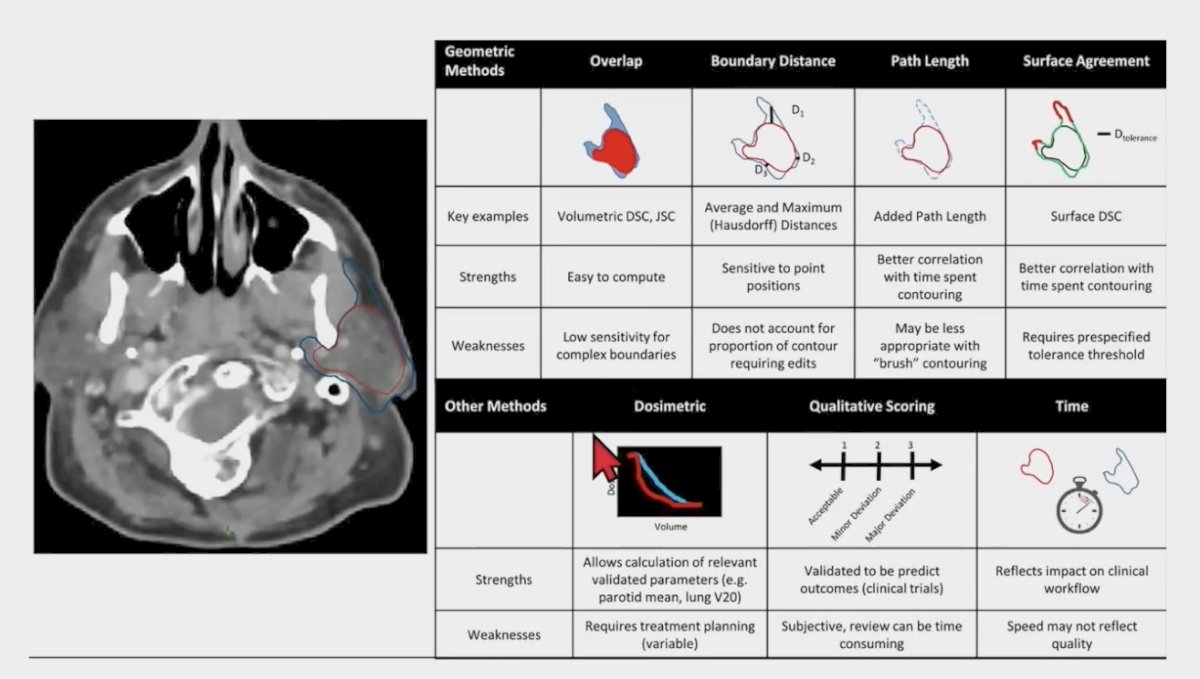
One study has assessed the implementation of deep learning-based auto-segmentation for radiotherapy planning structures, with two DC models implemented at two cancer centers [1]. These were used to generate organs at risk and CTVs for all patients undergoing radiotherapy for CNS cancer, head and neck cancer, and prostate cancer. DCs were generated on 551 eligible cases and radiotherapy technicians, dosimetrists and radiation oncologists completed post-contouring surveys (32% overall compliance rate with survey completion). The majority of organ at risk DCs required minimal edits subjectively (mean editing score <= 2) and objectively (mean DSC and 95% HD was >= 0.90 and <= 2.0 mm). Furthermore, the mean organ at risk satisfaction score was 4.1 for CNS cancer, 4.4 for head and neck cancer, and 4.6 for prostate cancer.
Dr. Beriwal’s group has also evaluated their experience with deep image-to-image network auto-segmentation algorithm [2]. In their study, they evaluated 156 patients and 1,366 contours prospectively, with the 5 most commonly contoured organs including:
- Lung: 136 contours, average rating 4.0
- Spinal cord: 106 contours, average rating 3.1
- Eye globe: 80 contours, average rating 3.9
- Lens: 77 contours, average rating 3.9
- Optic nerve: 75 contours, average rating 4.0
Overall, the average rating per evaluator per contour was 3.6 and on average, 124 contours were evaluated by each evaluator. Only 4% of contours were rate as 1 or 2 and no organ had an average rating <3. Thus, they concluded that artificial intelligence segmentation performed well with greater than 95% of contours accepted by the treating physicians with no or only minor edits.
A subsequent study from Dr. Beriwal’s group looked at pelvic nodal auto-segmentation using a deep image to image network auto-segmentation algorithm comparing the male and female pelvis [3]. In this study, pelvic nodal auto-segmentation features were trained and developed in the male pelvis and then assessed in both male (n = 51) and female (n = 52) patients. Overall 96% (450 pelvic nodal contours) and 99% (443 pelvic nodal contours) required no or minor edits for female and male patients (p = 0.004). The right internal iliac was the only nodal group with a statistically significant difference between female (92% requiring no or minor edits) and male (100% requiring no or minimal edits) patients (p = 0.04). Overall, the percentage of patients requiring no or minor edits was 87% (45 patients) and 92% (47 patients) for female and male patients, respectively (p = 0.36). As such, pelvic nodal auto-segmentation performed very well in both male and female pelvic nodal regions.
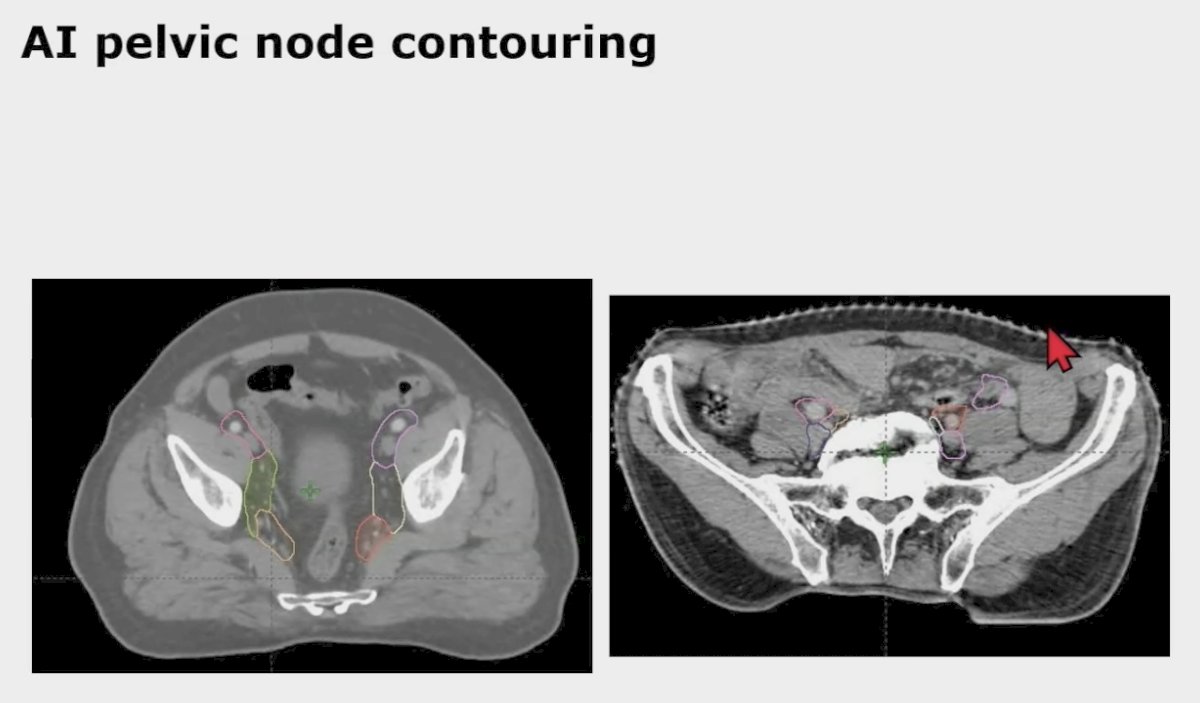
For treatment planning, this saves times, ensures homogeneity of plans, provides better coverage or sparing organs at risk, and can be used in clinical trials. However, one of the limitations is the inability to capture the abstract component of a radiation oncologist’s preferences/nuances.
Previously, McIntosh et al. [4] prospectively evaluated a random forest algorithm for therapeutic curative-intent radiation therapy treatment planning for prostate cancer in a blinded, head-to-head study with full integration into the clinical workflow:
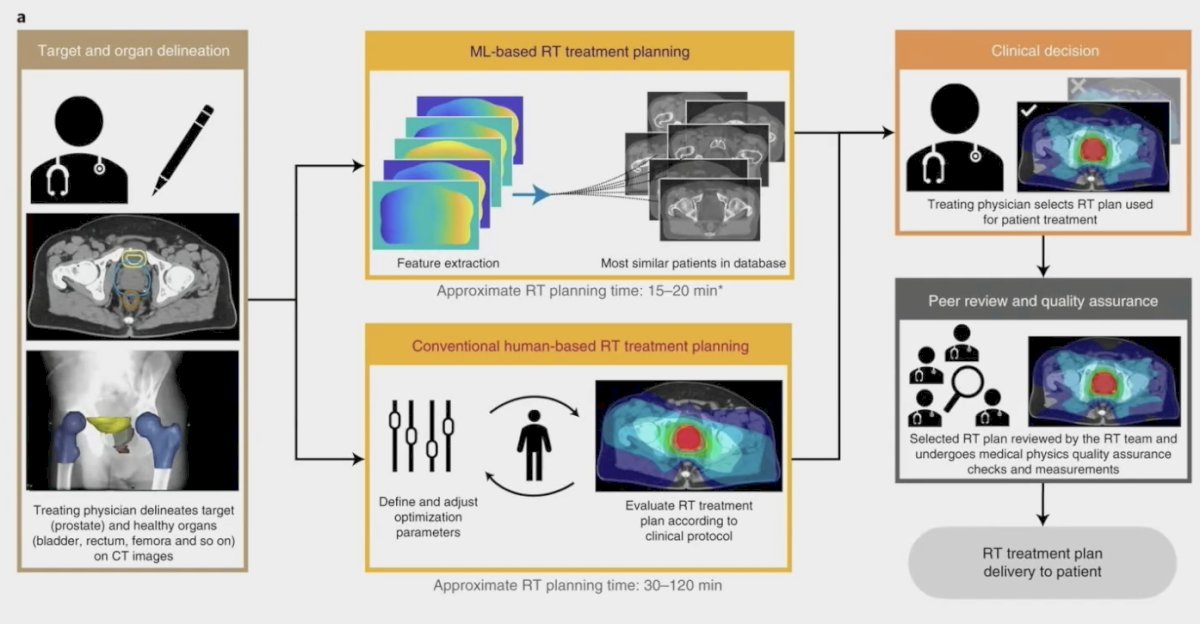
Overall, 89% of machine learning-generated radiotherapy plans were considered clinically acceptable and 72% were selected over human-generated radiotherapy plans in head-to-head comparisons. Additionally, radiotherapy planning using machine learning reduced the median time required for the entire radiotherapy planning process by 60.1% (118 hours to 47 hours). While machine learning radiotherapy plan acceptability remained stable between the simulation and deployment phases (92% versus 86%), the number of machine learning radiotherapy plans selected for treatment was significantly reduced (83% versus 61%, respectively).
As follows is an adaptive workflow and artificial intelligence:

Finally, Dr. Beriwal discussed the role of artificial intelligence in the development of a predictive biomarker for short term ADT benefit. Spratt and colleagues [5] used digital pathology images from pretreatment prostate tissue and clinical data from 5,727 patients enrolled in five phase 3 randomized trials, in which treatment was radiotherapy with or without ADT, as the data source to develop and validate an artificial intelligence–derived predictive patient-specific model that would determine which patients would develop the primary end point of distant metastasis:
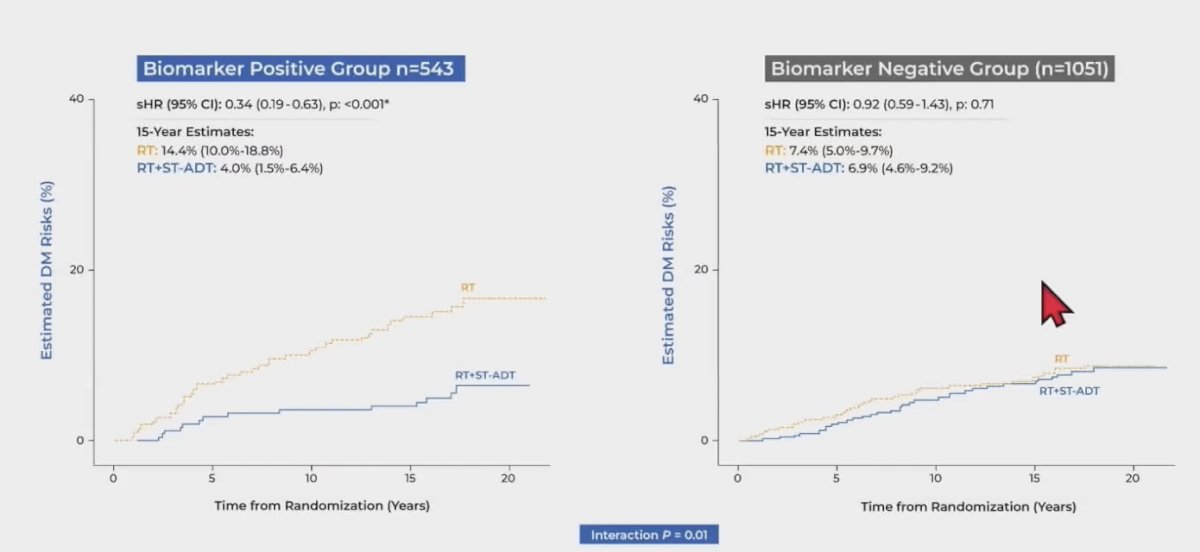
In the NRG/RTOG 9408 validation cohort, ADT significantly improved time to distant metastasis, and of these enrolled patients, 543 (34%) were artificial intelligence-model positive, with ADT significantly reduced the risk of distant metastasis compared with radiotherapy alone. However, there were 1,051 patients who were artificial intelligence-model negative whereby ADT did not provide benefit:
There are several challenges associated with artificial intelligence in radiotherapy:
- The training and data set is critical: because artificial intelligence/deep learning are data driven approaches, its success is heavily dependent on the data available to construct, optimize, and evaluate models. We need to make sure data is representative in terms of demographics, stage, and disease type
- Deep learning-based image segmentation models may be dependent on CT vendor: models from images acquired from the CT scanner from one vendor may not deliver accurate results when applied to images from a scanner of a different vendor
- DNN models can inherently be unstable when small changes in the model input may drastically alter the output
Dr. Beriwal concluded his presentation discussing artificial intelligence tools in radiation oncology clinical practice with the following take-home messages:
- Artificial intelligence in radiation therapy is still in the early phase, but rapidly progressing
- There is a potential to improve care, make it faster, more efficient and homogeneous
- Auto-segmentation, treatment planning and quality assurance are already being used
- Artificial intelligence has made adaptive plans and treatment feasible
- Physicians have a responsibility to see if the artificial intelligence performs in clinical practice
- The dataset used to generate and validate the model may influence its performance
- Regulatory pathways for approval are different for predictive or practice changing applications
Presented by: Sushil Beriwal, MD, Allegheny Health Network, Pittsburgh, PA
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2023 American Society for Therapeutic Radiation Oncology (ASTRO) 65th Annual Meeting held in San Diego, CA between October 1st and 4th, 2023
References:
- Wong J, Huang V, Wells D, et al. Implementation of deep learning-based auto-segmentation for radiotherapy planning structures: A workflow study at two cancer centers. Radiat Oncol. 2021 Jun 8;16(1):101.
- Beriwal S, et al. Evaluation of a deep image-to-image network (DI2IN) auto-segmentation algorithm across a cancer center. JCRT. 2023
- Beriwal S, et al. Pelvic nodal auto-segmentation using a deep image to image network (DI2IN) auto-segmentation algorithm: Comparing male vs female pelvis. ARO. 2023
- McIntosh C, Conroy L, Tjong MC, et al. Clinical integration of machine learning for curative-intent radiation treatment of patients with prostate cancer. Nat Med. 2021 Jun;27(6):999-1005.
- Spratt DE, Tang S, Sun Y, et al. Artificial Intelligence Predictive Model for Hormone Therapy Use in Prostate Cancer. NEJM Evid 2023;2(8).


