(UroToday.com) The 2023 ASTRO annual meeting included a session on patient-reported quality of life in prostate cancer, featuring a presentation by Dr. Gregory Alexander discussing conditional risk and predictive factors associated with late toxicity for prostate cancer patients treated with external beam radiotherapy alone in RTOG 0126. Given that patients with localized prostate cancer treated with radiotherapy alone have excellent outcomes, making quality of life and minimization of toxicities of great concern. The risk of developing toxicity may evolve with time from treatment which is of interest to patients seen in follow up. The primary objective of this study is to determine the conditional risk of grade 2+ gastrointestinal or genitourinary toxicity for patients treated with radiotherapy in the RTOG 0216 trial, which was a prospective randomized trial that randomized men with localized prostate cancer to two different radiotherapy schedules. A secondary objective was to determine risk factors associated with the development of late toxicities.
Dr. Alexander and colleagues performed a post-hoc analysis of RTOG 0126, which enrolled patients with intermediate risk prostate cancer who were randomized to receive standard dose radiotherapy of 70.2 Gy in 39 fractions vs dose escalated dose radiotherapy of 79.2 Gy in 44 fractions. The trial design for RTOG 0216 is as follows:
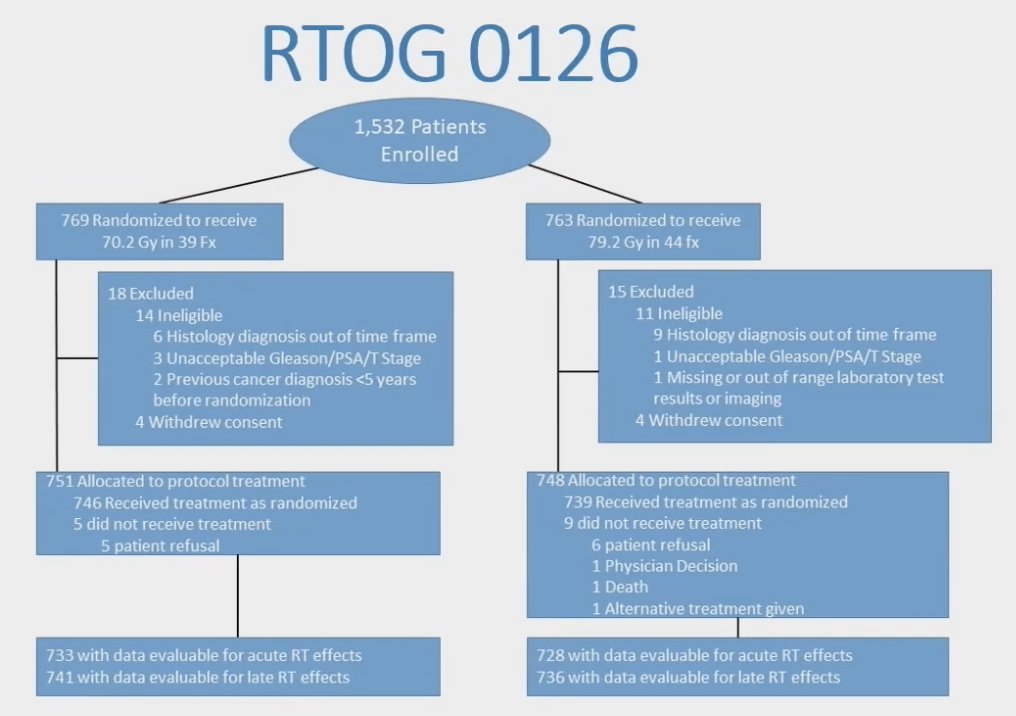
Cumulative incidence was used to calculate risk of grade 2+ genitourinary and grade 2+ gastrointestinal toxicity at initial time points, as well as for patients who survived 2 years and 5 years without respective grade 2+ toxicity. Risk factors of age, race, urinary incontinence, baseline urinary frequency, acute genitourinary toxicity, treatment arm, radiotherapy method (3DCRT vs IMRT), and organ at risk contouring protocol violation were analyzed using univariate and multivariate Cox proportional hazards modeling for late genitourinary toxicity. Risk factors of age, race, radiotherapy method, acute gastrointestinal toxicity, dose escalated dose radiotherapy, radiotherapy method, and organ at risk contouring protocol violation were analyzed for late gastrointestinal toxicity. There were 1,499 patients with a median follow up of 8.4 years (range 0.02-13 years) included in this analysis. The conditional cumulative 5-year risk of late grade 2+ gastrointestinal toxicity at enrollment, 2-, and 5-years of grade 2+ gastrointestinal toxicity free survivorship was 14.7%, 6.2%, and 1.8% respectively. The cumulative 5-year risk of late grade 2+ genitourinary toxicity at enrollment, 2-, and 5-years of late grade 2+ genitourinary toxicity free survivorship was 7.9%, 4.7%, and 2.7% respectively:

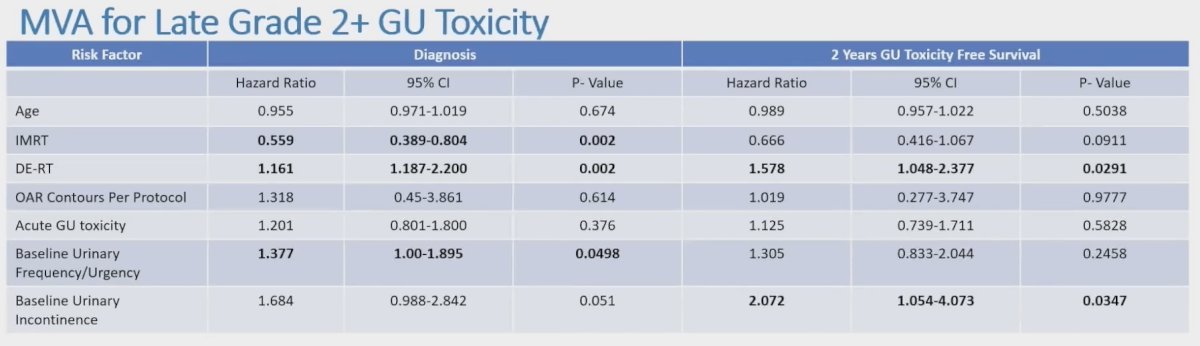
Dose escalated dose radiotherapy (HR 1.616, 95% CI 1.187-2.2, p < 0.002), IMRT (HR 0.559, 95% CI 0.389-0.804, p = 0.002), and urinary frequency at baseline (HR 1.377, 95% CI 1.00-1.895 p = 0.0498) were predictive of late genitourinary toxicity at enrollment on multivariable analysis:
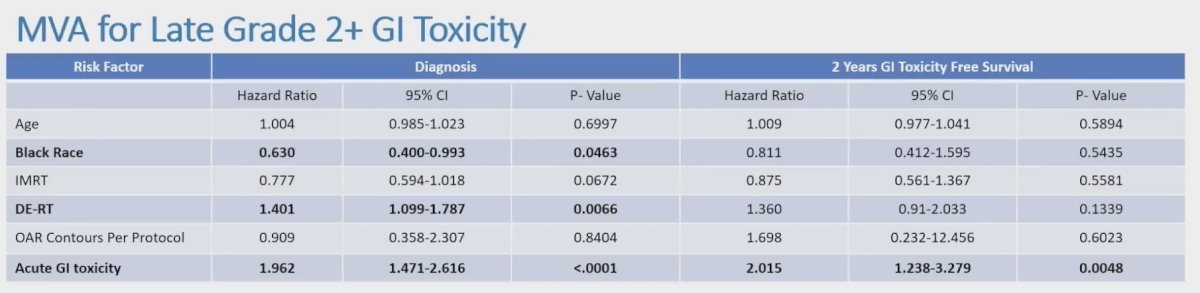
Initially dose escalated dose radiotherapy (HR 1.401, 95% CI 1.099-1.787; p = 0.0066), acute gastrointestinal toxicity (HR 1.962, 95% CI 1.471-2.616; p < 0.0001), and black race (HR 0.630, 95% CI 0.400-0.993; p = 0.0463) were predictive of late gastrointestinal toxicity. After two years of 2+ gastrointestinal toxicity free survivorship, only acute gastrointestinal toxicity (HR 2.015, 95% CI 1.238-3.279, p = 0.0048) remained predictive of late gastrointestinal toxicity on multivariable analysis:
Dr. Alexander concluded his presentation discussing conditional risk and predictive factors associated with late toxicity for prostate cancer patients treated with external beam radiotherapy alone in RTOG 0126 with the following take-home messages:
- The conditional risk of grade 2+ gastrointestinal and genitourinary toxicities decrease with time, with the majority of toxicities occurring the first 5 years
- Baseline urinary dysfunction and dose escalated radiotherapy were associated with increased late genitourinary toxicity, while IMRT was associated with decreased late toxicity
- Acute gastrointestinal toxicity and dose escalated radiotherapy were associated with increased late gastrointestinal toxicity, while black race was associated with decreased late toxicity
- This data provides a framework for the appropriate length of clinical follow-up required for prospective trials where the primary goal is side effect reduction
- This data can also be used to help inform patients seen in clinic during both initial consultation and routine follow-up
Presented by: Gregory Alexander, MD, University of Maryland, Baltimore, MD
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2023 American Society for Therapeutic Radiation Oncology (ASTRO) 65th Annual Meeting held in San Diego, CA between October 1st and 4th, 2023


