The 2023 ASTRO annual meeting included a session on patient-reported quality of life in prostate cancer, featuring a presentation by Dr. Simon Spohn discussing toxicity and patient reported quality of life after PSMA-PET and mpMRT-based focal dose escalated definitive radiotherapy in prostate cancer patients.
For patients that recur after radiotherapy, most local recurrences occur in the dominant intraprostatic lesions. As such, focal dose escalation yields isotoxic improvement in outcomes based on data from the FLAME trial:1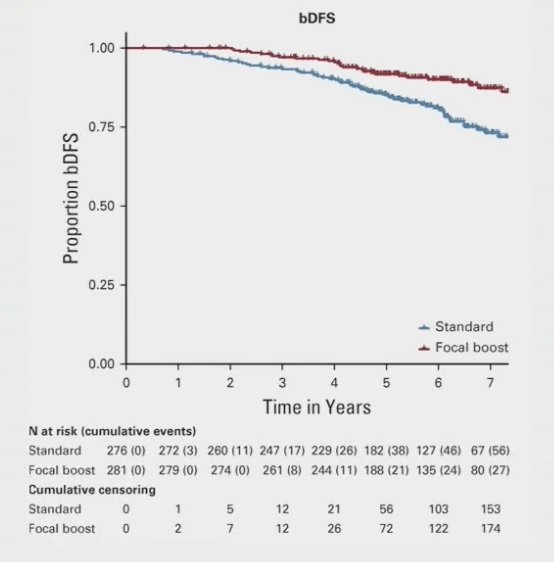
Furthermore, MRI underestimates the true tumor volume and may be associated with high interobserver variability: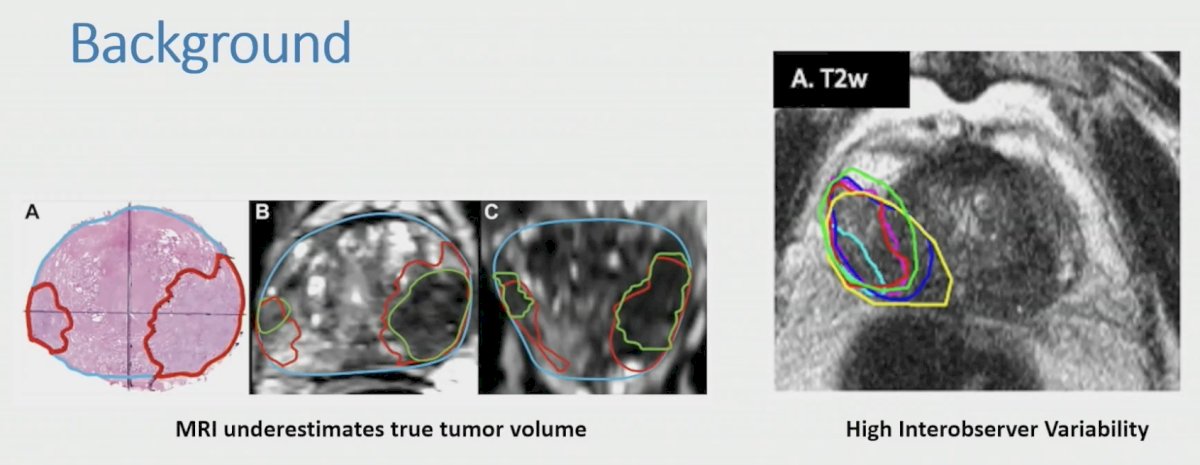
PSMA PET/CT has been suggested as a more accurate modality for depicting the true tumor volume. The prospective, 2-armed non-randomized HypoFocal phase II trial investigated the safety and feasibility of focal dose escalated external beam radiotherapy and high-dose-rate brachytherapy for prostate cancer patients based on PSMA-PET and multiparametric MRI. This approach improves tumor coverage and thus putatively treatment effectiveness but leads to larger boost volumes. At the 2023 ASTRO annual meeting, Dr. Spohn and colleagues presented toxicity and patient reported quality of life results after 2 years follow-up.
Patients with intermediate- or high-risk prostate cancer and cN0/cM0 stage were included in this trial. Patients in arm A received 60 Gy in 20 fractions to the prostate with an integrated boost of up to 75 Gy, and patients in arm B received one session of high-dose-rate brachytherapy with 15 Gy to the prostate and a boost of up to 19 Gy, followed by external beam radiotherapy of 44 Gy in 20 fractions. Boost volumes were defined by PSMA-PET and mpMRI based on validated approaches. The trial design for HypoFocal is as follows:
Genitourinary and gastrointestinal toxicity (CTCAE v5.0) and quality of life with IPSS and EORTC questionnaires (QLQ30 and PR25) were assessed. There were 50 patients treated in both arms in two centers (Freiburg and Berlin). The baseline characteristics are as follows: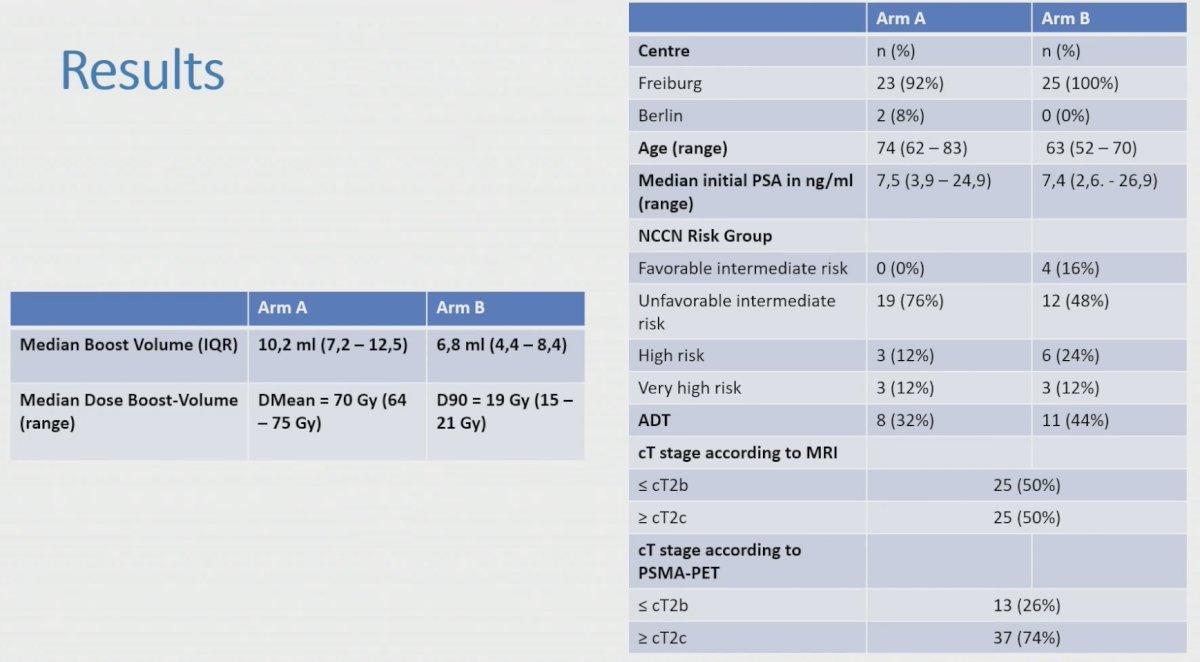
In arm, A grade 2 genitourinary and gastrointestinal toxicity rates after 2 years were 8% and 4%. There were no grade 3 genitourinary toxicities. Two patients experienced grade 3 gastrointestinal toxicities due to multifactorial causes. In Arm B grade 2 genitourinary and gastrointestinal toxicity rates after 2 years were 17% and 0%. No grade 3 toxicities were observed in arm B. Toxicities were not statistically significantly different between baseline and 2 year follow-up (p>0.055):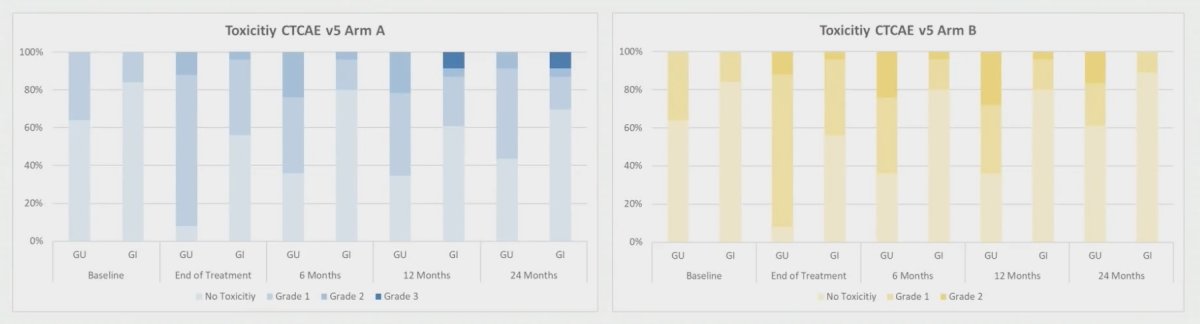
Quality of life analysis was performed with patients with available questionnaires at baseline and 2 year follow-up (12-15 in Arm A and 13-15 in Arm B). Only bowel function (p = 0.0005, median 4.2 vs 25 points) in Arm A and sexual- (p = 0.004, median 25 vs 50 points) and bowel function (p = 0.003, median 0 vs 8.3 points) and dyspnea (p = 0.031, median 0 vs 0 points) in Arm B decreased significantly after 2 year follow-up. Other quality of life items were not significantly different. Bowel symptoms were significantly worse in Arm A compared to Arm B (p = 0.003). Median PSA values after 2 years were 0.23 ng/ml in Arm A and 0.33 ng/ml in Arm B.
Dr. Spohn concluded his presentation discussing toxicity and patient reported quality of life after PSMA-PET and mpMRT-based focal dose escalated definitive radiotherapy in prostate cancer patients with the following take-home messages:
- The 2 year follow-up of the HypoFocal-Phase II trials shows no significantly increased genitourinary and genitourinary toxicities compared to baseline symptoms
- Patients reported about a good quality of life but increased bowel symptoms after 2 years, particularly if treated with external beam radiotherapy only
- Implementation of PSMA-PET into focal dose escalated radiotherapy approaches appears safe and feasible, despite large boost volumes
- Radiation proctitis demands careful and interdisciplinary management
Presented by: Simon Spohn, MD, University Medical Center Freiburg, Freiburg, Germany
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2023 American Society of Radiation Oncology (ASTRO) Annual Meeting, San Diego, CA, Sun, Oct 1 – Wed, Oct 4, 2023.
References:


