(UroToday.com) The 2024 American Society for Radiation Oncology (ASTRO) annual meeting held in Washington D.C., was host to the session Presidential Symposium: Innovations in Genitourinary Cancers: Session II - Bladder Preservation - A Modern Choice for Patients. Dr. Gopakumar Iyer discussed how to Integrate Novel Therapeutics with trimodal therapy (TMT).
Radiation therapy (RT) has substantial immunomodulatory effects in the tumor microenvironment. RT-induced cell death leads to the release of immunogenic damage-associated molecular patterns (DAMPs) and activates several inflammatory pathways, resulting in immune cell activation. However, RT can also cause lymphopenia, which may lead to immunosuppression. Therefore, optimizing the timing and dose of radiation delivery is crucial, as these factors significantly influence the immunomodulatory effects within the tumor and the overall response to RT.
Dr. Iyer mentioned that we need to learn from our colleagues treating lung cancer. Recently, the PACIFIC study, a phase III trial comparing durvalumab with placebo in patients with unresectable, stage III non–small-cell lung cancer (NSCLC) and no disease progression after concurrent chemoradiotherapy (CRT), showed significant improvements in overall survival (OS) and progression-free survival (PFS). This study confirmed that CRT followed by durvalumab had a profound effect on these patients, highlighting the potential benefits of integrating immunotherapy with RT in other cancer types as well.1
The Keynote 992 RCT phase 3 is precisely examining the combination of immune checkpoint inhibitor (ICI) with trimodal therapy (TMT), in this case, Pembrolizumab vs. placebo in patients with muscle-invasive bladder cancer (MIBC) (T2-T4 N0M0) opting for bladder-preservation receiving concurrent CRT. The radiation therapy (RT) was the investigators' choice of conventional RT (64 Gy at 2 Gy/fraction over 6.5 weeks to the whole bladder +/- pelvic nodes) or hypofractionated RT (55 Gy at 2.75 Gy/fraction over 4 weeks to the whole bladder only). This was given along with radiosensitizing cisplatin or 5-fluorouracil (5-FU)/mitomycin-C (MMC) or gemcitabine. The primary endpoint of the study was bladder-intact event-free survival (BIEFS) (defined as residual/recurrent MIBC, nodal or distant metastasis, radical cystectomy (RC), or death from any cause). Secondary endpoints include OS, metastasis-free survival (MFS), time to non-muscle-invasive bladder cancer (NMIBC) occurrence, time to RC, and safety. This study is ongoing and hopes to complete accrual by May 2027.
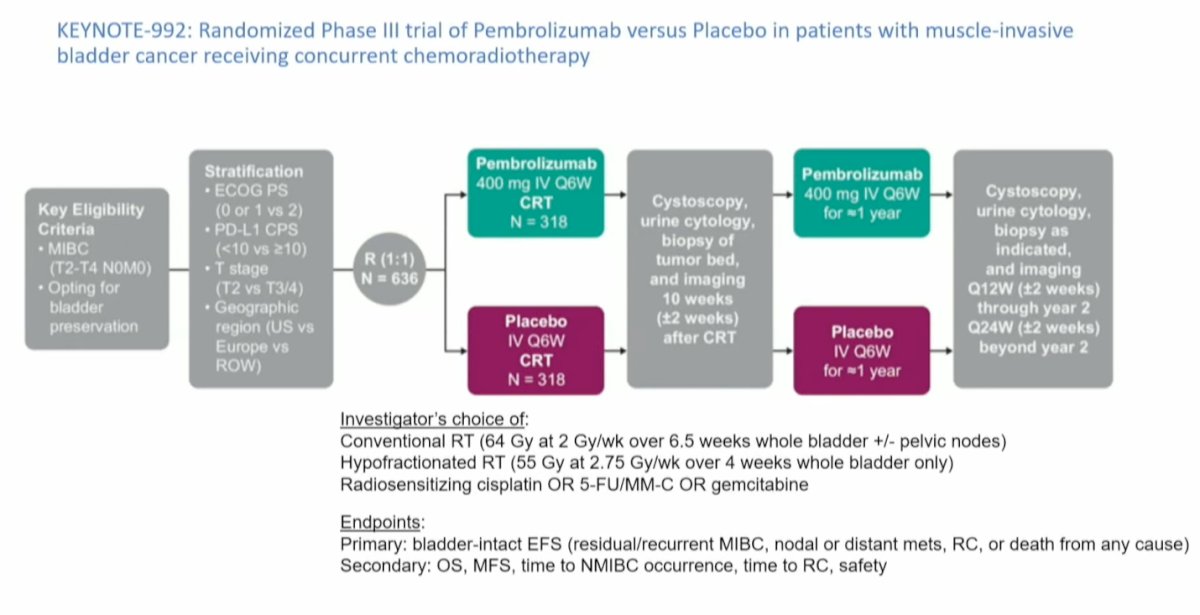
Similarly, the Tour de Force study SWOG S1806 enrolled patients with clinical T2-T4N0M0 bladder cancer and randomized them (n=475) to receive either chemoradiotherapy (CRT) alone or CRT combined with Atezolizumab for 9 cycles. In this study, radiation therapy (RT) was delivered as 64 Gy at 2 Gy per fraction for 32 fractions or 64 Gy at 1.8 Gy per fraction for 36 fractions; unfortunately, hypofractionation was not allowed. The primary endpoint, similar to the Keynote 992 trial, is bladder-intact event-free survival (BIEFS). We are eagerly awaiting the results of this trial.
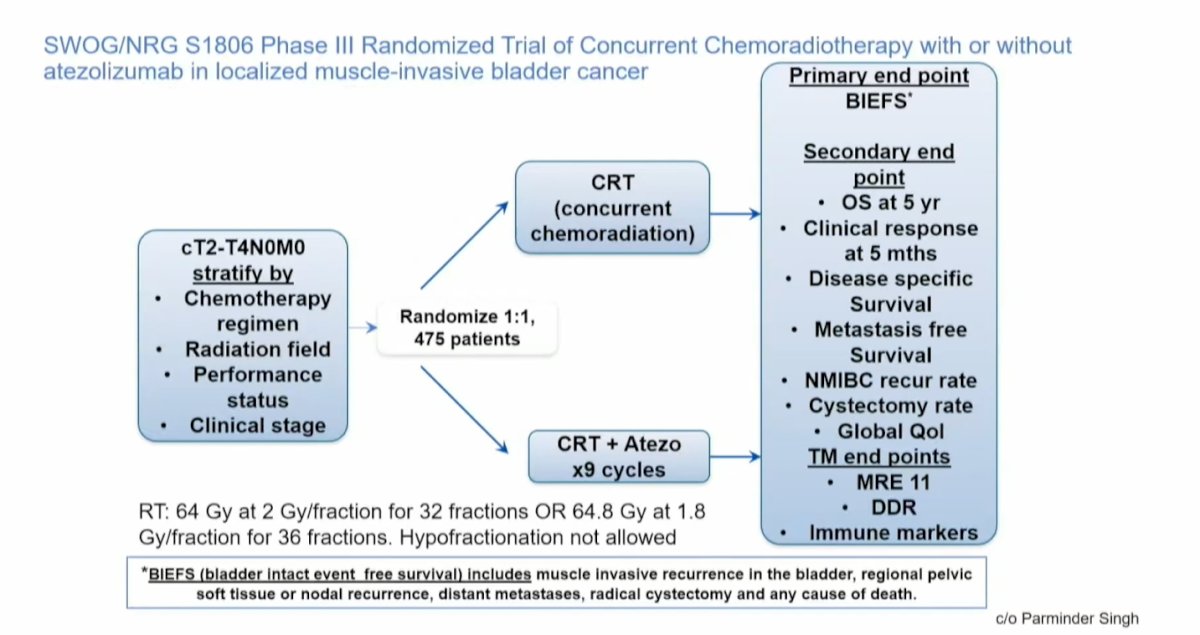
Pivoting to antibody-drug conjugates (ADCs), enfortumab vedotin (EV) has emerged as a major player in the locally advanced/metastatic urothelial carcinoma (la/mUC) setting. EV works by binding to receptors on the surface of cancer cells. It is internalized into the cell, where it releases a cytotoxic compound, monomethyl auristatin E (MMAE), which causes microtubular disruption and cell cycle arrest, leading to direct tumor cell apoptosis. Additionally, MMAE is released into the extracellular space, killing more cancer cells.
The EV-302 trial has changed the standard of care (SOC) in the first-line setting for metastatic urothelial carcinoma, comparing gemcitabine and cisplatin (GC) to EV combined with pembrolizumab. The trial showed impressive results with a progression-free survival (PFS) hazard ratio (HR) of 0.45 and an overall survival (OS) HR of 0.47, making EV + pembrolizumab the de facto first-line treatment for mUC to date.2 The question we now face is whether we can move this treatment earlier in the disease course.
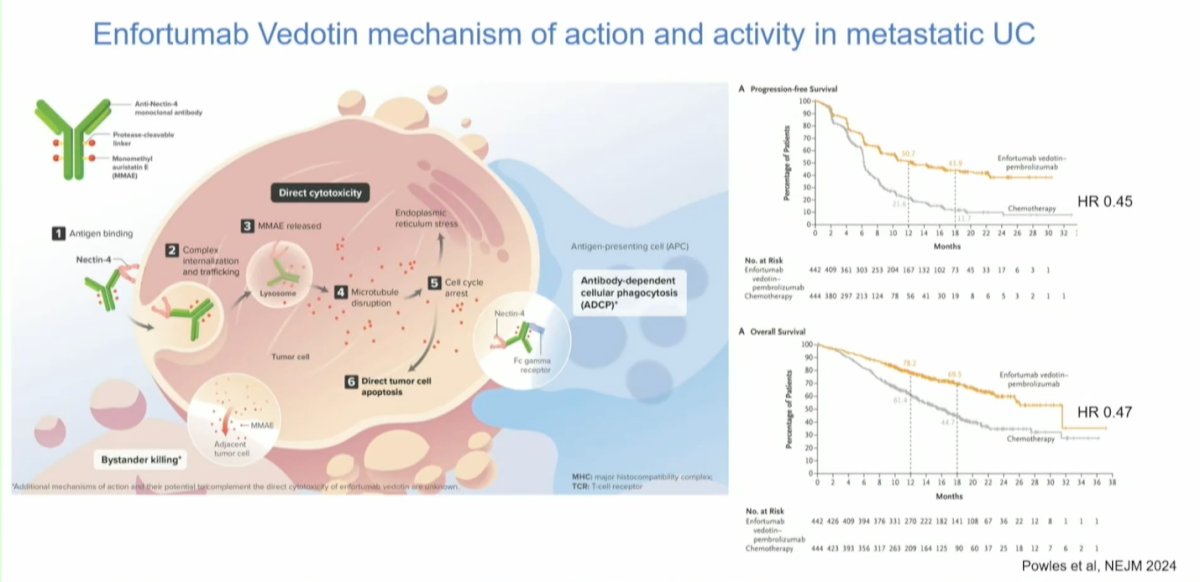
The EV-103 study focused on a cohort of patients who were ineligible for cisplatin. The EV-103 Cohort H study explored neoadjuvant EV in cisplatin-ineligible patients with MIBC in this trial patients are receiving 3 cycles of neoadjuvant EV followed by RC. Notably, 86% of patients completed EV therapy, and all patients underwent radical cystectomy (RC) without delays due to EV toxicity. Impressively, the pathological complete response (pCR) rate was 36.4%, and the pathological downstaging (pDS) rate was 50%. Surgery-related grade ≥3 treatment-emergent adverse events (TEAEs) occurred in 31.8% of patients, but the toxicity profile was similar to that of cisplatin-based chemotherapy.3 This trial has laid the foundation for launching two large phase 3 randomized controlled trials (RCTs).
The ongoing trials include EV-303, which compares EV plus pembrolizumab versus pembrolizumab alone versus upfront RC in cisplatin-ineligible patients. The EV-304 trial compares EV plus pembrolizumab versus gemcitabine and cisplatin followed by RC in cisplatin-eligible patients. The results of these trials are eagerly awaited. However, it's important to note that none of these trials are in the bladder-sparing space; they are assessing antibody-drug conjugates +/- ICI before patients undergo RC.
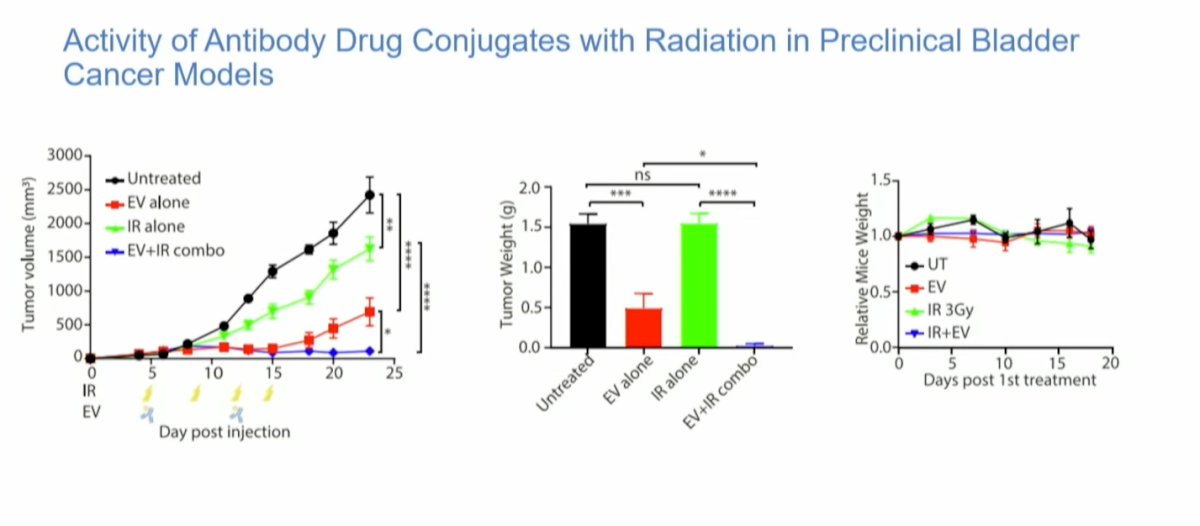
Preclinical data from studies combining antibody-drug conjugates with RT in mice have shown promising results. Mice treated with RT alone or in combination with EV demonstrated significant reductions in tumor volume and tumor weight. Notably, there was minimal weight loss in the mice, suggesting relatively low toxicity. This data supports the potential for combining ADCs with RT in treating cancer while maintaining a manageable safety profile.
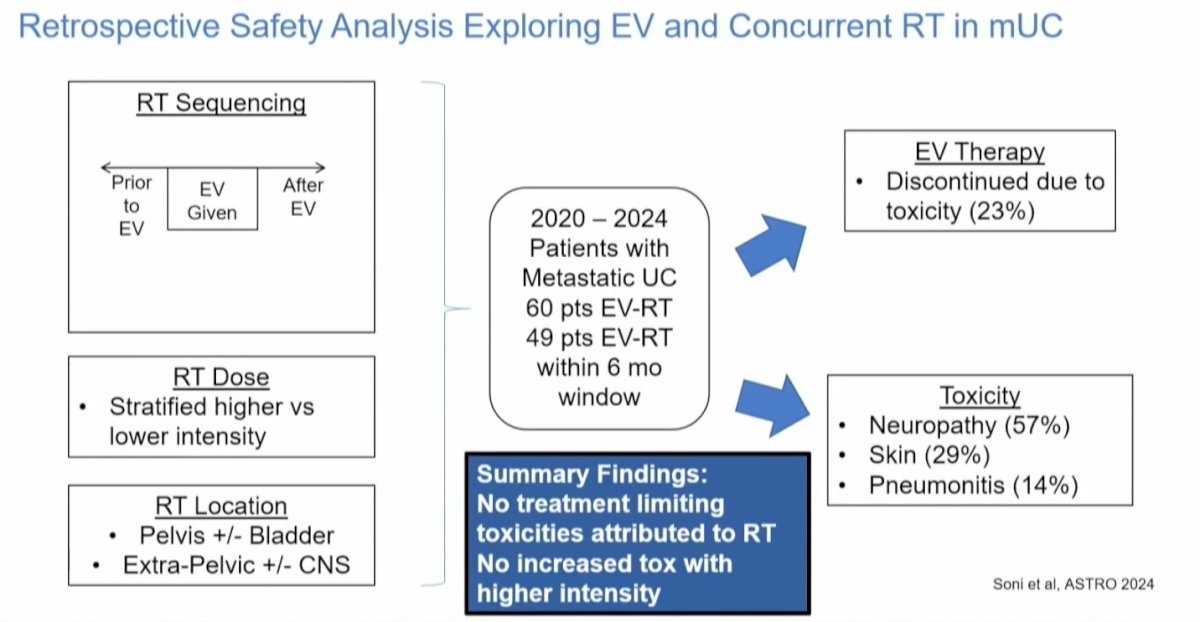
A retrospective safety analysis of 109 patients with metastatic urothelial carcinoma who received RT in conjunction with EV is set to be presented at the upcoming ASTRO conference. Notably, only 23% of patients discontinued EV therapy due to toxicity. The analysis indicated that toxicity was not exacerbated by the location or intensity of RT, and no treatment-limiting toxicities were attributed to RT. Moreover, higher intensity did not correlate with increased toxicity. The most common adverse events reported were neuropathy (57%) and skin-related adverse events (29%), which are typically associated with EV therapy. These findings suggest that EV can be safely combined with RT.
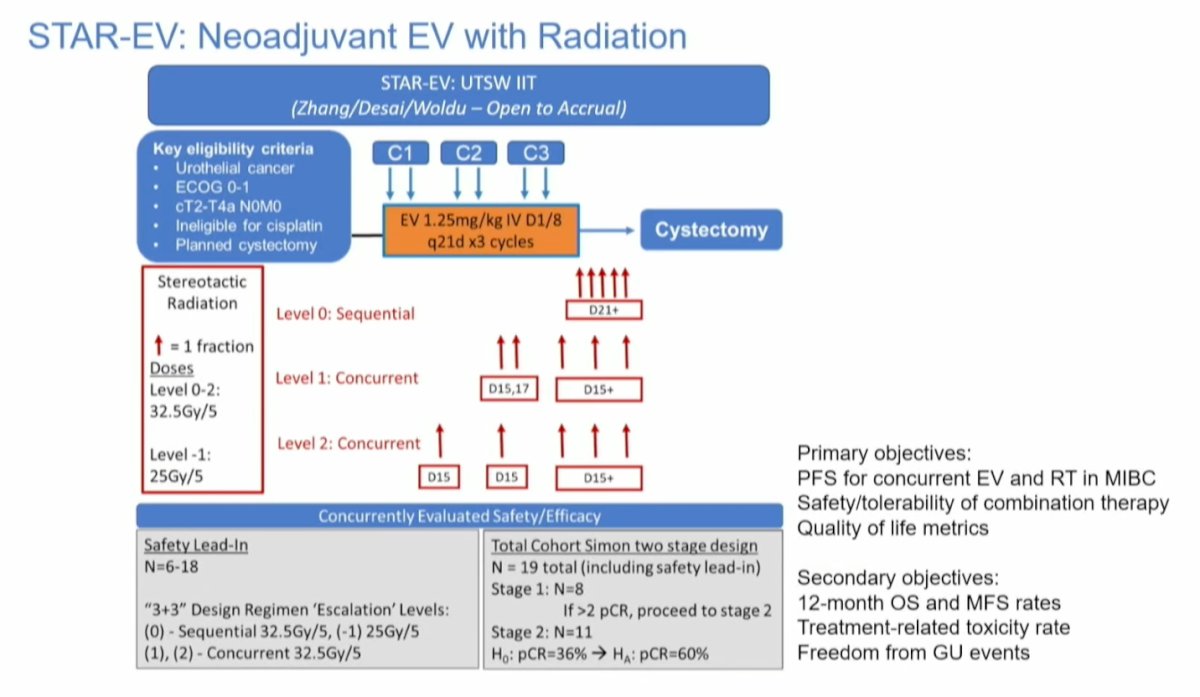
The STAR-EV trial is a single center, phase 1/2 trial based at UT Southwestern Medical Center. Patients with cT2-T4N0M0 urothelial cancer ineligible for cisplatin who are planned for RC will receive EV for 3 cycles, with either sequential or concurrent stereotactic body radiation (SBRT) in 5 fractions. The safety lead-in phase will consist of SBRT given at C3 day 21 and then escalated forward to start at C2 day 15 (level 1) or C1 day 15 (level 2). All patients will then undergo radical cystectomy. The primary objective is PFS for concurrent EV+RT followed by RC. The study design is shown below:
Dr. Iyer moved on to discuss the role of predictive biomarkers in bladder preservation strategies. As shown in the graphic below, there are multiple predictive biomarkers; however, he focused on two: ERCC2 and circulating tumor DNA (ctDNA).

ERCC2 (Excision Repair Cross-Complementation Group 2) is a gene that encodes a protein involved in the nucleotide excision repair (NER) pathway, which is essential for DNA damage repair (DDR). Mutations in ERCC2 have been linked to improved outcomes following chemoradiotherapy and higher pathologic complete response (pCR) rates at RC after NAC. This association is likely due to its role in conferring sensitivity to cisplatin-based chemotherapy, enhancing the efficacy of treatment in patients with bladder cancer.
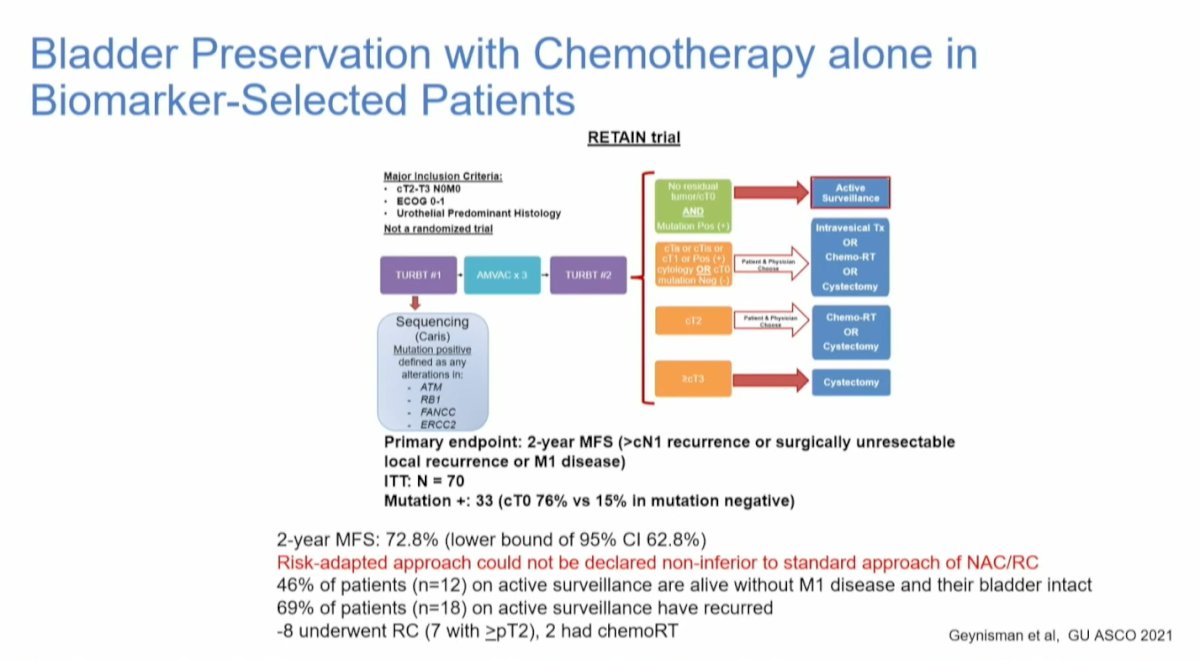
The RETAIN trial enrolled patients with clinical T2-T3N0M0, predominantly urothelial carcinoma. After undergoing transurethral resection of bladder tumor (TURBT) and genetic sequencing of the tumor for DDR gene alterations, patients received a combination of gemcitabine and cisplatin (AMVAC) followed by a second TURBT. For those achieving a complete response (CR) defined as clinical T0, negative urine cytology, and imaging without residual disease, the study offered either active monitoring alone or RC. This trial was the first to utilize chemotherapy alone in biomarker-selected patients.
The study completed accrual, and the 2-year metastasis-free survival was reported at 72.8% (lower bound of 95% confidence interval [CI] 62.8%). However, this risk-adapted approach could not be declared non-inferior to the standard approach of neoadjuvant chemotherapy followed by radical cystectomy (NAC/RC). At the last follow-up, 46% of patients (n=12) remained on active surveillance, alive without M1 disease, and with their bladders intact. Among those on active surveillance, 69% (n=18) experienced recurrence; of these, 8 underwent RC (7 with ≥pT2), while 2 received CRT.4
Another study examining bladder preservation following chemotherapy in biomarker-selected patients is the Alliance study (A031701). This study is still in the process of accruing participants, and the study diagram is shown below:
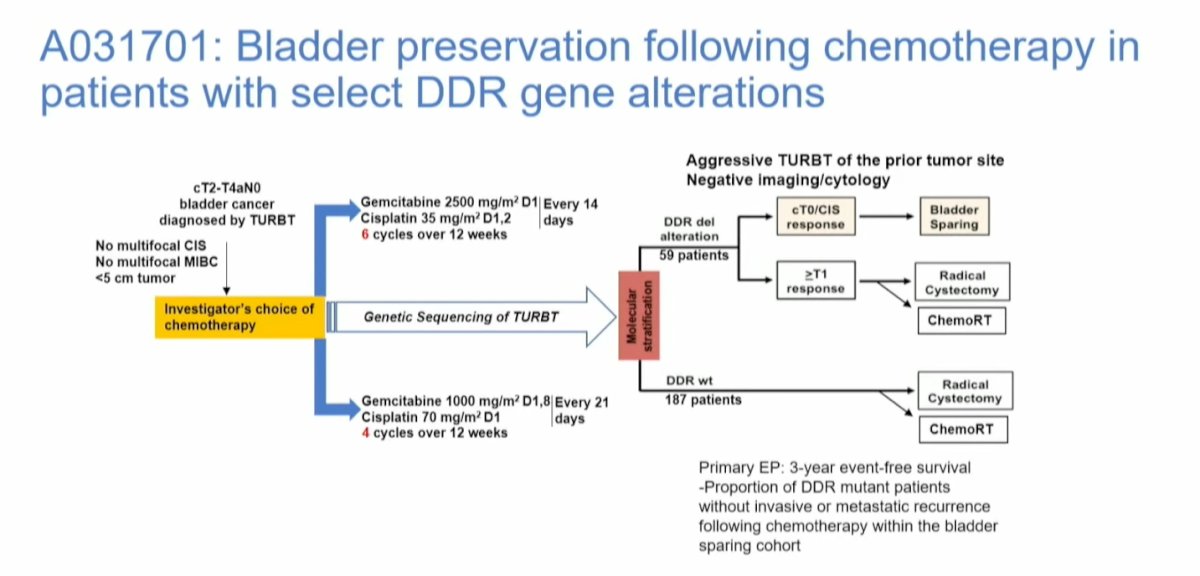
In a study analyzing genomic and transcriptomic features linked to long-term responses in patients with muscle-invasive bladder cancer (MIBC) treated with trimodal therapy (TMT), pretreatment tumors from 76 patients underwent whole-exome sequencing, and 67 matched transcriptomic profiling. The results showed that tumor mutational burden was not associated with TMT outcomes; however, alterations in DNA damage response genes were linked to improved locoregional control and modified bladder-intact event-free survival (mBI-EFS). Notably, somatic mutations in the ERCC2 gene were significantly associated with favorable outcomes, demonstrating improved BIEFS when compared to those without ERCC2 mutations.5

ctDNA has been identified as a predictive biomarker for long-term survival and recurrence after radical cystectomy (RC). In the IMvigor 010 trial, which compared adjuvant Atezolizumab to observation in patients with high-risk MIBC no significant overall benefit was observed with adjuvant Atezolizumab. However, when stratifying patients based on ctDNA status, those with positive ctDNA showed significantly worse recurrence-free survival (p<0.001) compared to those with negative ctDNA. This significant difference in disease-free survival was consistent for patients treated with adjuvant Atezolizumab as well as those who were under observation. Recently this was also observed in OS and ctDNA positivity identified patients with an OS benefit favoring atezolizumab versus observation (HR 0.59; 95% CI 0.42-0.83).6
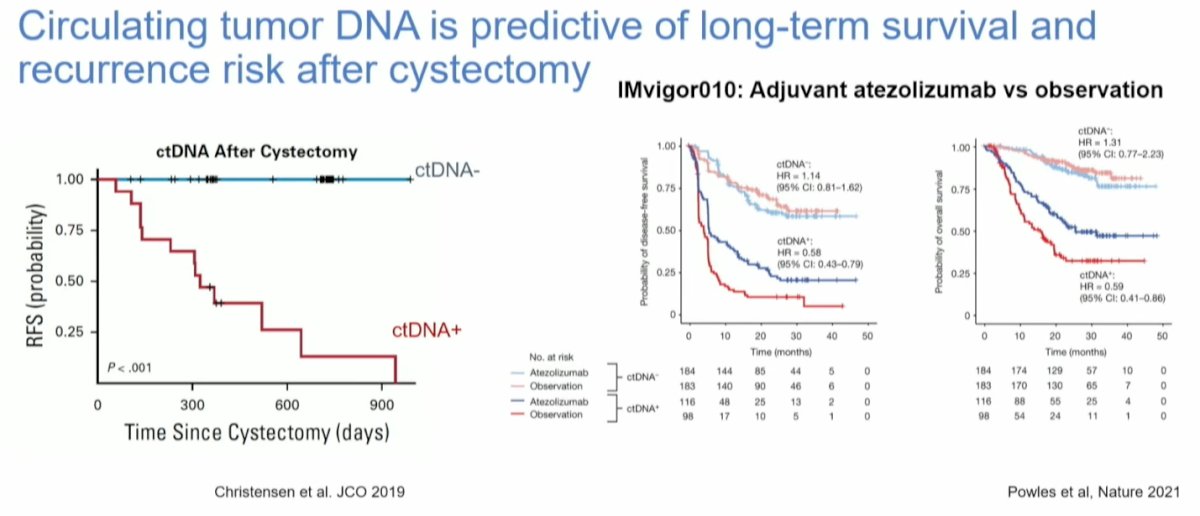
Additionally, data presented at ASTRO by Mouw et al. demonstrated a strong correlation between the persistence of ctDNA and an increased risk of recurrence after RT. Notably, 3 out of 4 patients with positive ctDNA status experienced recurrence with metastatic UC.

Dr Iyer, Wrapped up his presentation with the following take home-messages:
- Novel systemic therapies have transformed the treatment landscape in both advanced and organ-confined urothelial carcinoma (ICI, ADCs)
- Many trials are investigating bladder sparing without CRT in the MIBC space and results are awaited.
- We need to re-evaluate the importance/relevance of TMT in this new era: toxicity of prolonged systemic adjuvant therapy, financial toxicity, importance of local definitive therapy to urothelium
- Several trials are exploring combination ICI or ADCs with TMT in patients with MIBC
- Predictive biomarkers of response to TMT are being recognized and need validation through incorporation into prospective clinical trials (ctDNA and ERCC2 alterations are promising)
Written by: Julian Chavarriaga, MD – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @chavarriagaj on Twitter during the 2024 American Society for Radiation Oncology (ASTRO) annual meeting held in Washington D.C., between the 29th of September and the 2nd of October.
References:- David R. Spigel et al., Five-Year Survival Outcomes From the PACIFIC Trial: Durvalumab After Chemoradiotherapy in Stage III Non–Small-Cell Lung Cancer. JCO 40, 1301-1311(2022). DOI:10.1200/JCO.21.01308
- Powles, Thomas, et al. "Enfortumab vedotin and pembrolizumab in untreated advanced urothelial cancer." New England Journal of Medicine 390.10 (2024): 875-888.
- Peter H. O'Donnell et al. Study EV-103: Neoadjuvant treatment with enfortumab vedotin monotherapy in cisplatin-ineligible patients with muscle invasive bladder cancer (MIBC)—2-year event-free survival and safety data for Cohort H.. JCO 42, 4564-4564(2024).
- Daniel M. Geynisman et al. A phase II trial of risk-enabled therapy after initiating neoadjuvant chemotherapy for bladder cancer (RETAIN).. JCO 41, 438-438(2023).
- Kamran SC, Zhou Y, Otani K, Drumm M, Otani Y, Wu S, Wu CL, Feldman AS, Wszolek M, Lee RJ, Saylor PJ, Lennerz J, Van Allen E, Willers H, Hong TS, Liu Y, Davicioni E, Gibb EA, Shipley WU, Mouw KW, Efstathiou JA, Miyamoto DT. Genomic Tumor Correlates of Clinical Outcomes Following Organ-Sparing Chemoradiation Therapy for Bladder Cancer. Clin Cancer Res. 2023 Dec 15;29(24):5116-5127. doi: 10.1158/1078-0432.CCR-23-0792. PMID: 37870965; PMCID: PMC10722135.
- Powles T, Assaf ZJ, Degaonkar V, Grivas P, Hussain M, Oudard S, Gschwend JE, Albers P, Castellano D, Nishiyama H, Daneshmand S, Sharma S, Sethi H, Aleshin A, Shi Y, Davarpanah N, Carter C, Bellmunt J, Mariathasan S. Updated Overall Survival by Circulating Tumor DNA Status from the Phase 3 IMvigor010 Trial: Adjuvant Atezolizumab Versus Observation in Muscle-invasive Urothelial Carcinoma. Eur Urol. 2024 Feb;85(2):114-122. doi: 10.1016/j.eururo.2023.06.007. Epub 2023 Jul 26. PMID: 37500339.


