The 2024 ASTRO annual meeting included a session on the management of small cell neuroendocrine tumors of the genitourinary tract, featuring a presentation by Dr. Jean Hoffman-Censits discussing emerging systemic therapy and clinical trials. Dr. Hoffman-Censits notes that small-cell bladder and prostate cancer patients are typically excluded from clinical trials due to their aggressive biology and clinical behavior.
Looking at the NCCN guidelines for bladder cancer, it is noted that for both neoadjuvant chemotherapy and metastatic chemotherapy, guidance is provided by the “Principles of Systemic Therapy in NCCN Guidelines for Small Cell Lung Cancer.” For prostate cancer, the NCCN guidelines emphasize that these patients may be treated with cytotoxic chemotherapy (ie. cisplatin + etoposide, carboplatin + etoposide, docetaxel + carboplatin, or cabazitaxel + carboplatin). Additionally, physicians should also consult the NCCN guidelines for small cell lung cancer for additional options in the first and subsequent lines of therapy because the behavior of small cell/neuroendocrine carcinoma of the prostate is similar to that of small cell carcinoma of the lung.
Dr. Hoffman-Censits then discussed the data supporting the use of first-line platinum based chemotherapy for small cell bladder and prostate cancers. In a recent SEER analysis (2000-2020) from Ullah et al.1 of 1,040 patients with bladder small cell and large cell neuroendocrine carcinoma, they found that chemotherapy + local therapy improves overall survival over a single treatment modality alone: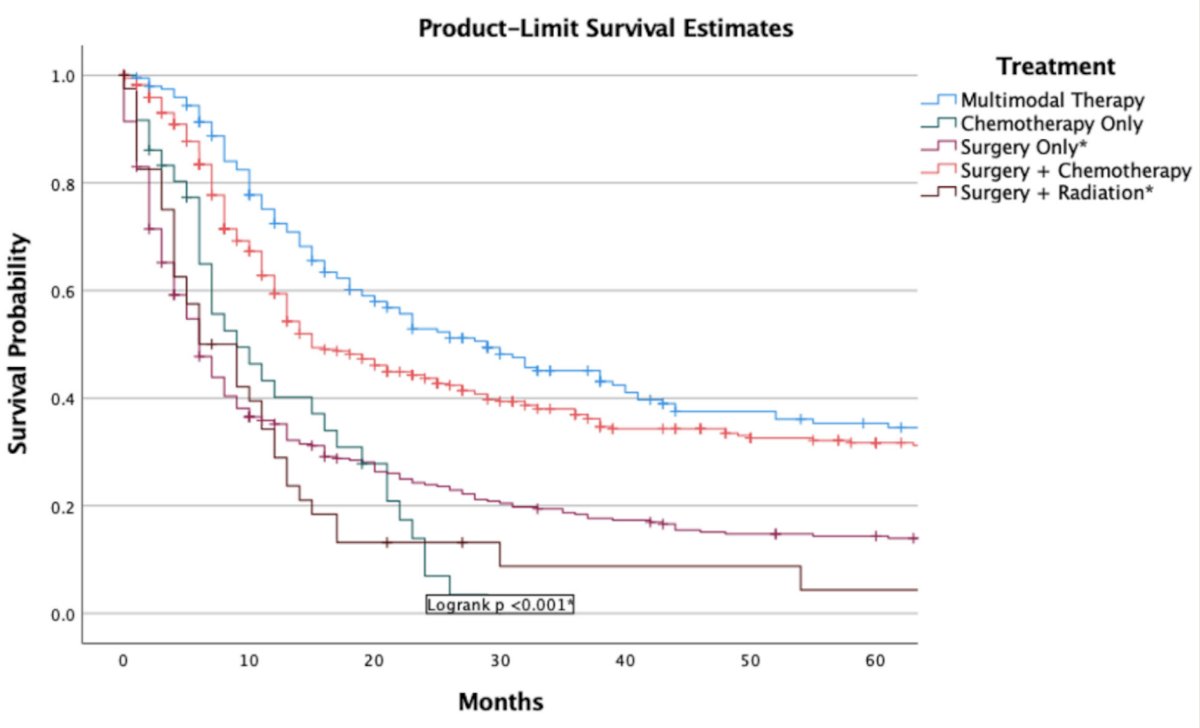
In another study comparing treatment strategies for bladder small cell carcinoma, Alhalabi et al.2 showed the importance of neoadjuvant chemotherapy versus adjuvant/no chemotherapy on overall survival:
Previous studies have suggested that etoposide regimens appear more effective than conventional urothelial cancer chemotherapy, with pathologic complete response rates of 41% for cisplatin + etoposide or carboplatin + etoposide compared to 25% for cisplatin + gemcitabine or carboplatin + gemcitabine or accelerated MVAC. Moreover, ERCC2 alterations appear to portend a better outcome, with these alterations more often associated with a pathologic complete response to neoadjuvant chemotherapy.
Specific to prostate cancer, Dr. Hoffman-Censits notes that men with small-cell prostate cancer typically present with symptoms, including voiding and obstruction. Furthermore, most men have CRPC or androgen-independent tumors, limiting the efficacy of primary ADT as well as using PSA as a biomarker. The majority of studies assessing chemotherapy for prostate small cell carcinoma have enrolled heterogeneous populations of small cell/variant histologies, generally showing that these tumors are chemosensitive, but with a short overall survival of 6-19 months.
Dr. Hoffman-Censits noted that when looking at the NCCN small cell lung cancer guidelines, first-line therapy for extensive-stage disease is chemotherapy in combination with atezolizumab or durvalumab, which perhaps may be extrapolated to small cell carcinomas of the genitourinary tract. Checkpoint inhibitors for small cell bladder cancer to date are limited to case reports and case series, with several treatment options and varying results:
While there is likely optimism for checkpoint inhibitors in small cell carcinoma of the bladder, Dr. Hoffman-Censits notes that neuroendocrine prostate cancer is generally characterized by a relatively immune-depleted tumor immune microenvironment. However, lurbinectedein and trastuzumab deruxtecan have been assessed in both prostate and bladder small cell carcinomas with mixed outcomes. Recently, in the metastatic urothelial carcinoma disease space, there has been great interest in enfortumab vedotin + pembrolizumab, however in an analysis of this combination in the UNITE dataset, there was a 0% objective response rate for patients with pure variants. This included a 0% objective response rate for any small cell component, regardless of percentage. Thus, Dr. Hoffman-Censits does not think enfortumab vedotin + pembrolizumab is a viable option for these patients at this point in time.
Dr. Hoffman-Censits finished her presentation by highlighting several ongoing trials of interest:
- First-line: Atezolizumab + etoposide and platinum chemotherapy in small cell bladder cancer
- NCT05312671
- Phase 2
- N = 63
- PI: Dr. Jean Hoffman-Censits
- Second line post-platinum chemotherapy: Lurbinectedin with or without avelumab in small cell carcinoma of the bladder (LASER)
- NCT06228066
- Phase II
- N = 35
- PI: Dr. Andrea Apolo
- Any line: Testing the effectiveness of two immunotherapy drugs (nivolumab + ipilimumab) with one anti-cancer targeted drug (cabozantinib) for rare genitourinary tumors (including prostate)
- NCT03866382
- Phase II
- N = 314
- PI: Dr. Andrea Apolo
- Any line: Sacituzumab govitecan with or without atezolizumab immunotherapy in rare genitourinary tumors (SMART) such as small cell, adenocarcinoma, and squamous cell bladder/urinary tract cancer, renal medullary carcinoma, and penile cancer
- NCT06161532
- Phase II
- N = 60
- PI: Dr. Andrea Apolo
Dr. Hoffman-Censits concluded her presentation discussing emerging systemic therapy and clinical trials with the following take-home points:
- Small cell cancers of the bladder (de novo) and prostate (mCRPC) are rare and aggressive tumors
- Treatment is extrapolated from the more common small cell lung cancer literature
- Approved therapies for small cell lung cancer may be hard to obtain in clinical practice
- Clinical trials are vital, but directed studies are currently small/single center experiences
- For trial design considerations, we need to include rare exploratory cohorts such as small cell carcinomas
- Checkpoint inhibitor indications are expanding in bladder cancer and may allow earlier use for these patients
Presented by: Jean Hoffman-Censits, MD, Assistant Professor of Oncology, Johns Hopkins University, Baltimore, MD
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Associate Professor of Urology, Georgia Cancer Center, Wellstar MCG Health, @zklaassen_md on Twitter during the 2024 American Society for Radiation Oncology (ASTRO) Annual Meeting, Washington, DC, Sun, Sept 29 – Wed, Oct 2, 2024.
References:
- Ullah A, Lee KT, Chaudhury H, et al. Prognostic Nomogram, Demographics and Comparative Analysis of Urinary Bladder Small Cell and Large Cell Neuroendocrine Carcinoma. Clin Genitourin Cancer. 2024 Jul 31;22(6):102183.
- Alhalabi O, Wilson N, Xiao L, et al. Comparative Effectiveness Analysis of Treatment Strategies for Surgically Resectable Neuroendocrine Carcinoma of the Urinary Tract. Eur Urol Oncol. 2023 6;611-620.


