(UroToday.com) The 2024 American Society for Radiation Oncology (ASTRO) Annual Meeting held in Washington, DC between September 29th and October 2nd, 2024, was host to a presidential symposium of innovations in genitourinary cancers, specifically addressing the ‘renaissance’ of radiotherapy for renal cell carcinoma (RCC). Dr. Shankar Siva discussed whether stereotactic ablative radiotherapy (SABR) for the treatment of primary RCC is ready for ‘primetime’.
Dr. Siva noted that there has been a worldwide increase in the incidence of RCC, from 7.1 cases per 100,000 in 1975 to 16 cases per 100,000 in 2008. Notably, this increase has been most rapid in the >70 years age group.1
The current standard of care treatment for primary RCC is surgery, ideally via a nephron-sparing approach, where technically feasible and oncologically safe. However, Dr. Siva noted that there are currently limited curative options for inoperable patients. While thermal ablation is an alternative, it has decreased efficacy when the tumor is >3–3.5 cm, and there is an increased incidence of complications for large masses. While thermal ablation does not require general anesthesia, it is not technically feasible for peri-hilar or large tumors and remains an invasive treatment modality.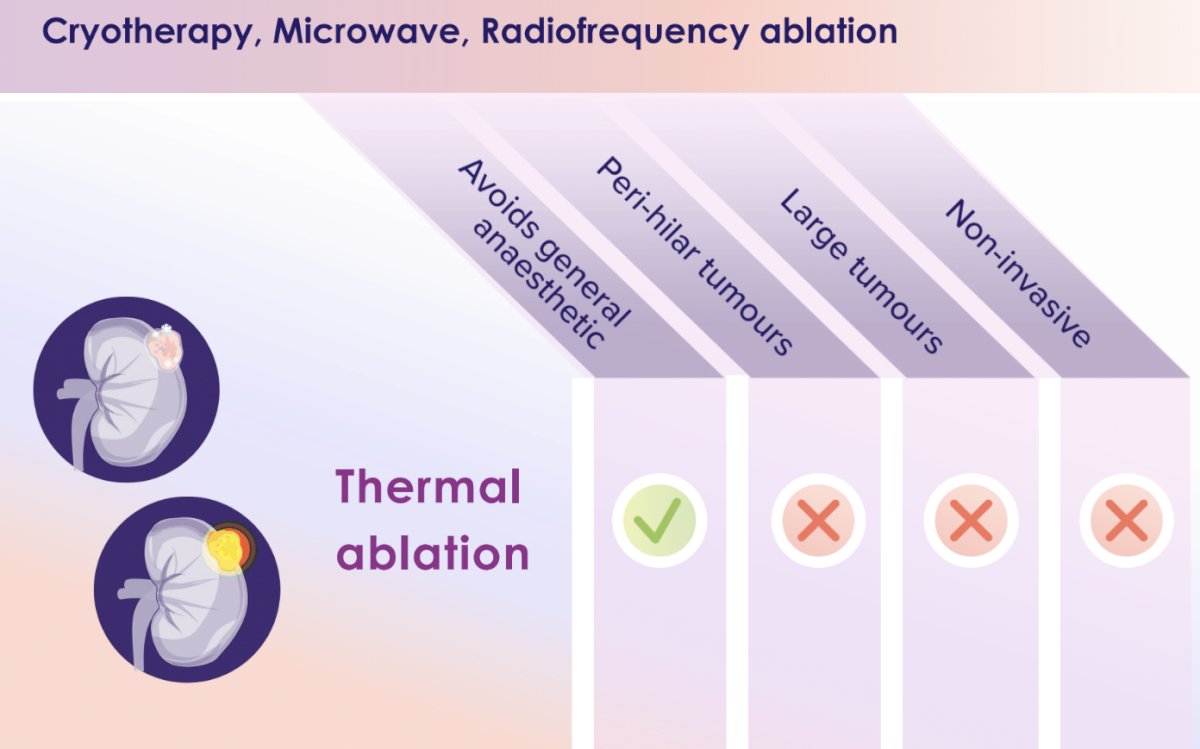
Dr. Siva argued that SABR may be an alternative option that ticks all of the boxes below: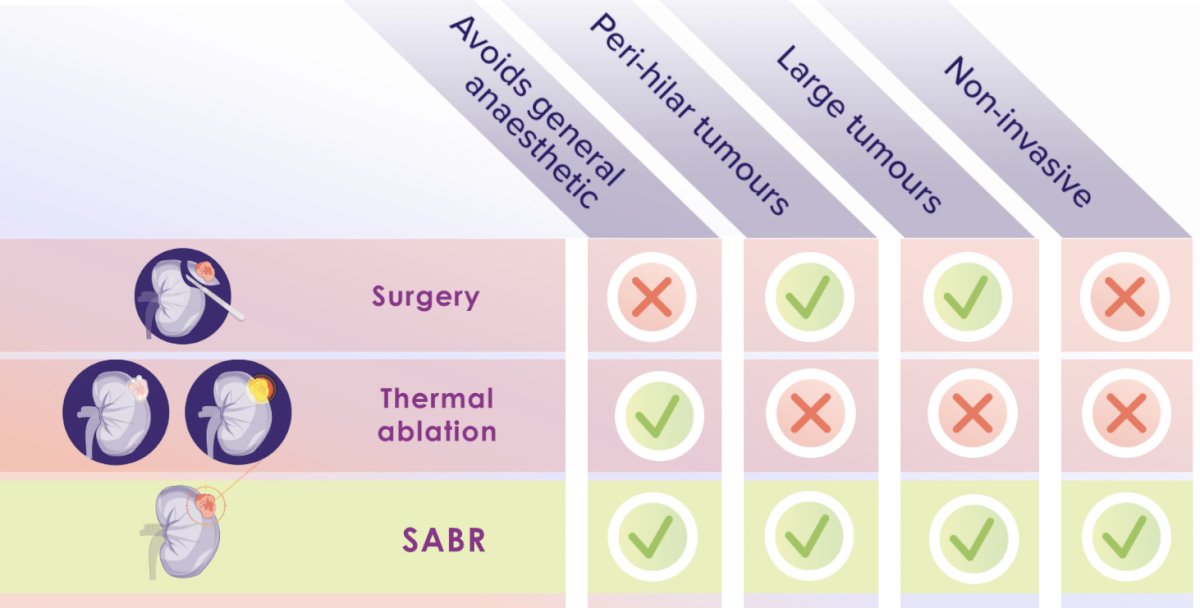
Historically, RCCs have been considered to be ‘radioresistant’. This hypothesis was supported by the results of previous trials that have demonstrated that the use of post-operative external beam radiotherapy may reduce the risk of locoregional failure but not overall survival.2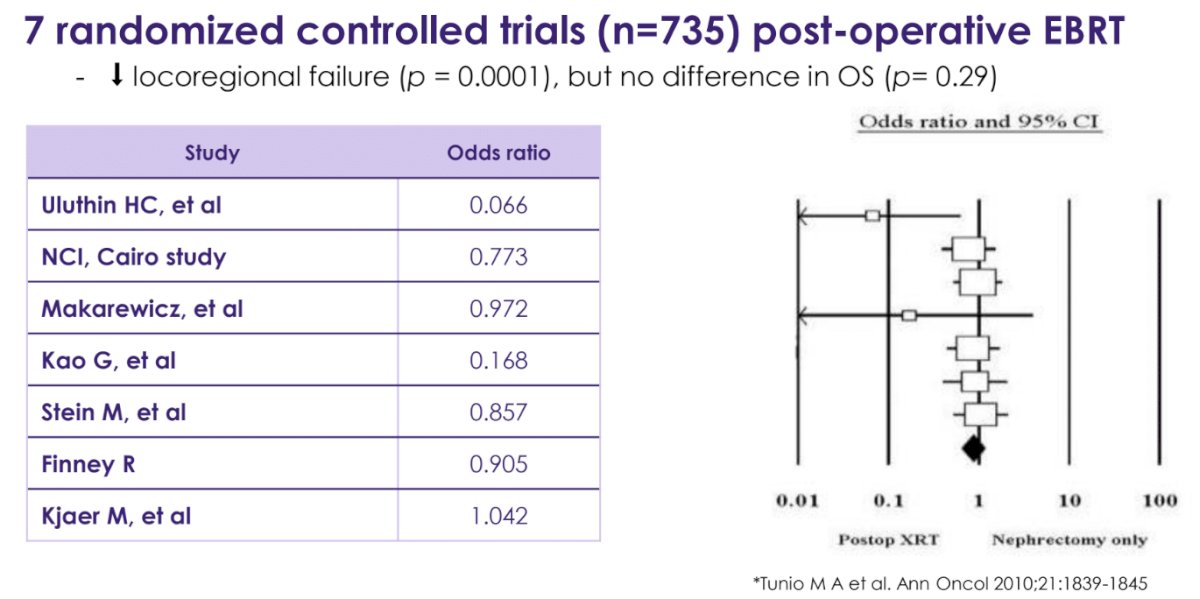
Furthermore, radiotherapy for RCC in the 1980s was associated with high rates of complications.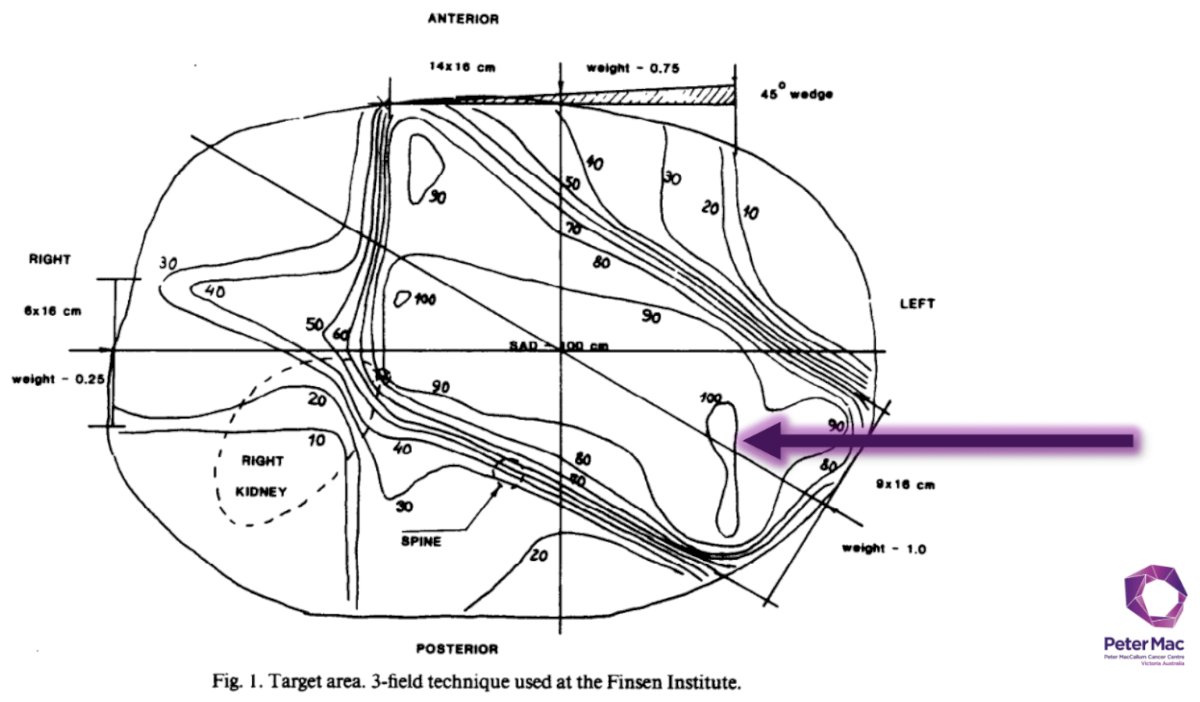
In 1997, radiobiologic studies of radioimmunotherapy and external beam radiotherapy in vitro and in vivo in human RCC xenografts demonstrated that there was small fraction of cell kills at doses of 2 Gy (i.e., radioresistant); however, there was evidence of logarithmic cell kill at doses >6 Gy (i.e., potentially radiosensitive).3
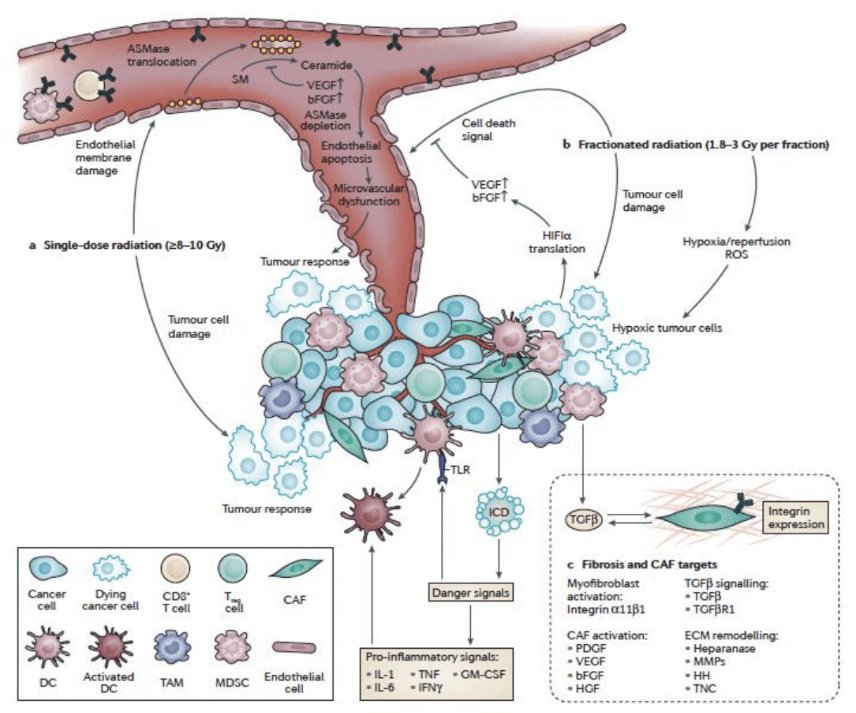
Potential mechanisms of cell killing with high-dose radiation include the following:
- Endothelial apoptosis
- Ceramide/sphingomyelinase-induced cell death
- Pro-inflammatory signaling for adaptive immunity
Illustrated below is the timeline of SABR for primary RCC. In 1995, fixed-frame SABR was used for the 1st time. In 2003, the first report of SABR targeting primary RCC tumors was published. This was followed by the 1st report of SABR for the treatment of primary RCC in patients with functionally solitary kidneys in 2008. In 2016, the 1st international consensus statement on SABR for primary RCC was released. This has all culminated in the publication of the results of the phase II FASTRACK II trial in The Lancet Oncology in 2024.4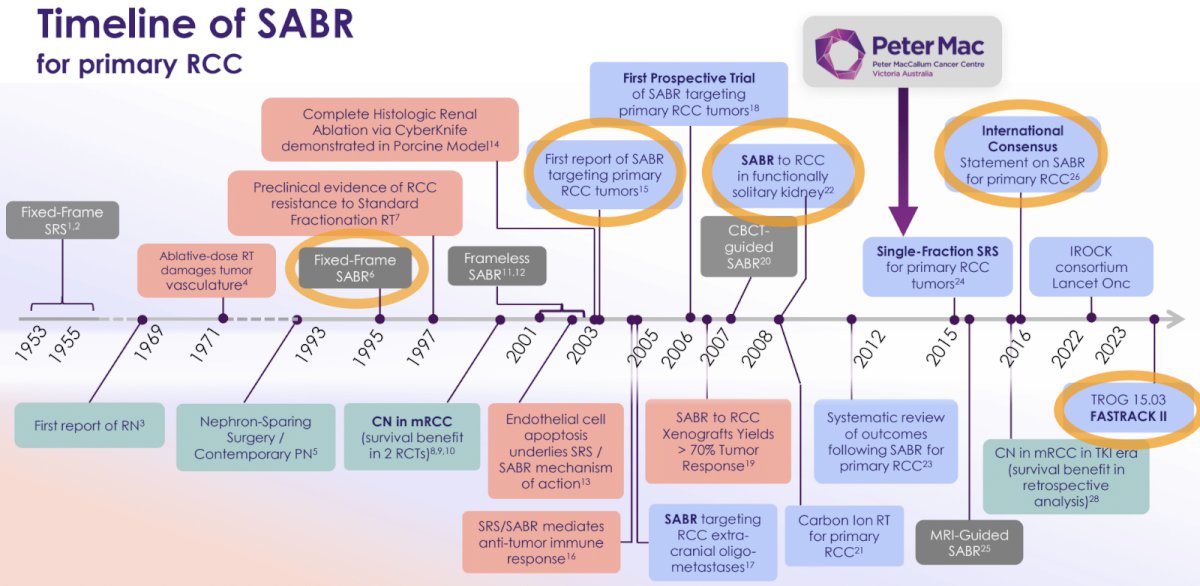
Since 2006, over a dozen prospective trials evaluating SABR for the treatment of primary RCC have been published. By comparison, there have been zero trials for thermal ablation during this timeframe. 265 patients have been enrolled in clinical trials of SABR for primary RCC. The efficacy outcomes are similar to those retrospectively reported for thermal ablation, even when accounting for the fact that there was zero re-treatment for SABR. Importantly, the renal function scores were similar to those observed with partial nephrectomies.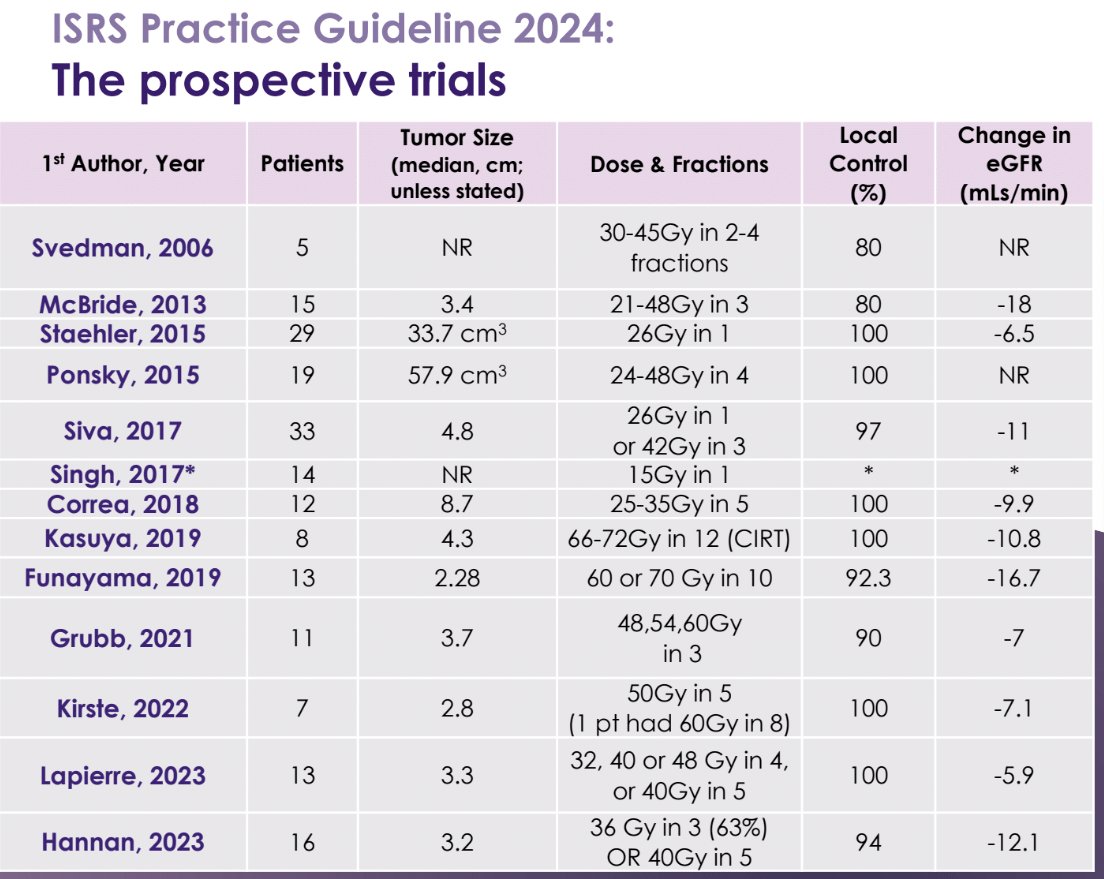
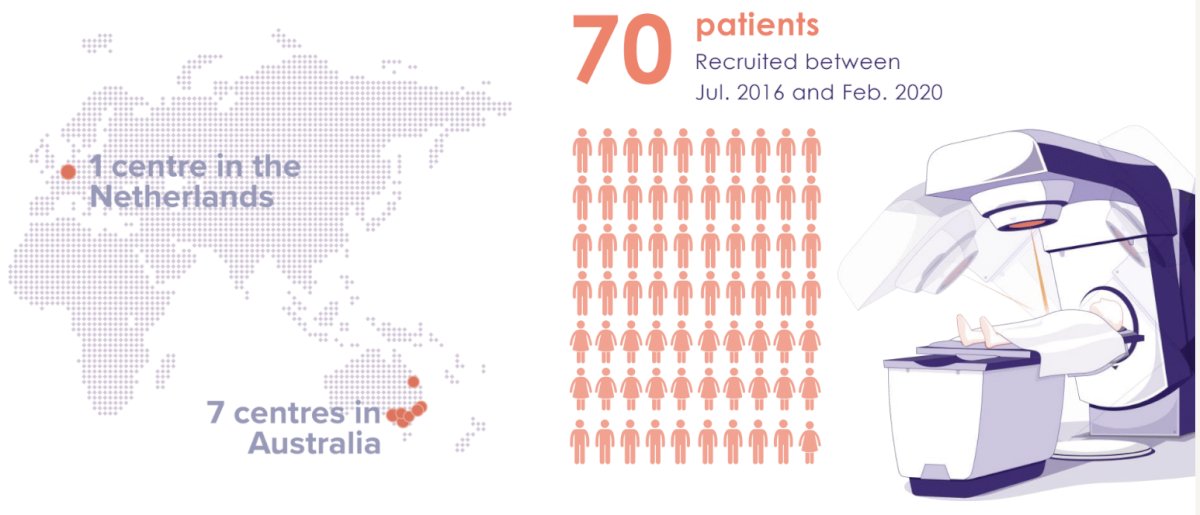
The FASTRACK II (Focal Ablative STereotactic Radiotherapy for Cancers of the Kidney) trial was a multicenter phase II trial that included patients with biopsy-confirmed RCC. The median tumor size was 4.6 cm, and 70% of the tumors had demonstrated evidence of serial growth at enrolment. Patients received SABR in 1 or 3 fractions.
Between July 2016 and February 2020, 70 patients were recruited from 7 centers in Australia and 1 in The Netherlands.
Eligible patients had to be deemed medically inoperable or high-risk for surgical complications and were recommended for active treatment via a multidisciplinary approach. All patients had an eGFR >30 ml/min. The maximum allowed tumor size was 10 cm, and the tumor was required to not be abutting the bowel.
The median patient age was 77 years, and the median BMI was 32 kg/m2. The median Charlson comorbidity index score was 7. The tumor stage was most commonly T1b (56%), followed by T1a (34%), T2a (95), and T3a (1%).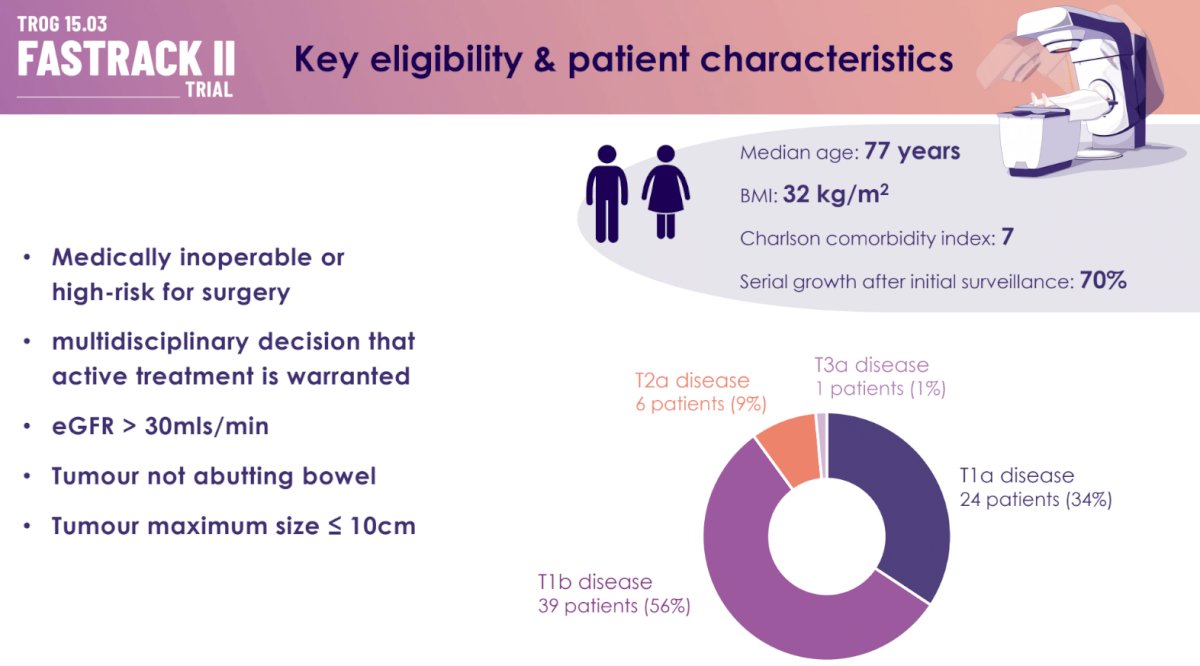
At a median follow-up of 43 months, the local control rate was 100%. 97% were free from distant failure, and none of the patients had experienced death secondary to their RCC. The mean kidney function loss was -14.6 mL/min, and only one patient underwent dialysis.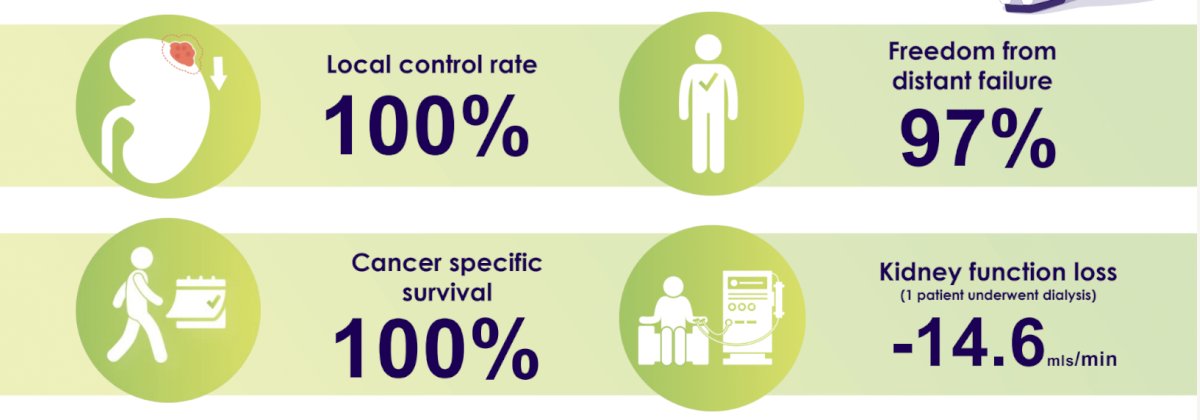
Are these results consistent with those from the IROCK (International Radiosurgery Consortium of the Kidney) individual patient data meta-analysis? In the 2022 report that included 190 patients with a median tumor size of 4.2 cm and who were followed for a median of 5 years, the cancer-specific survival was 92%. The distant failure rate was 10.8%, and the local failure rate was 5.5%. The mean eGFR loss was similar at 14.2 mL/min. Thus, we see that the results of the FASTRACK II trial ‘beat’ those of the IROCK report, which adds further support to the use of SABR for the treatment of primary RCC in clinical practice.
What are the key ‘enablers’ to reach primetime? Dr. Siva noted that we need to define the:
- Optimal dose regimen
- Role of post-treatment kidney biopsy
- Role of SABR in the solitary kidney
- Optimal post-treatment follow-up schedule
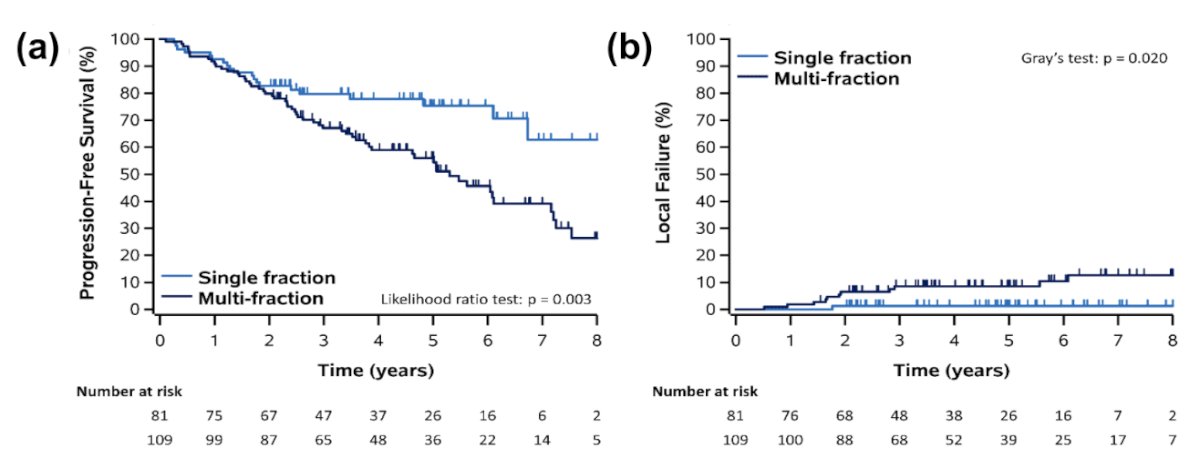
In an attempt to address the optimal dose regimen for SABR, Siva et al. demonstrated in 2022 that single fraction SABR (mostly 25–26 Gy) was superior to multi-fraction SABR for local failure and progression-free survival outcomes, both on univariable and multivariable analyses.5
Since 2012 at The Peter MacCallum Cancer Centre, patients with ≤4 cm targets have been receiving single fraction SABR (26 Gy), whereas those with >4 cm targets have been recommended for 42 Gy in 3 fractions, twice weekly.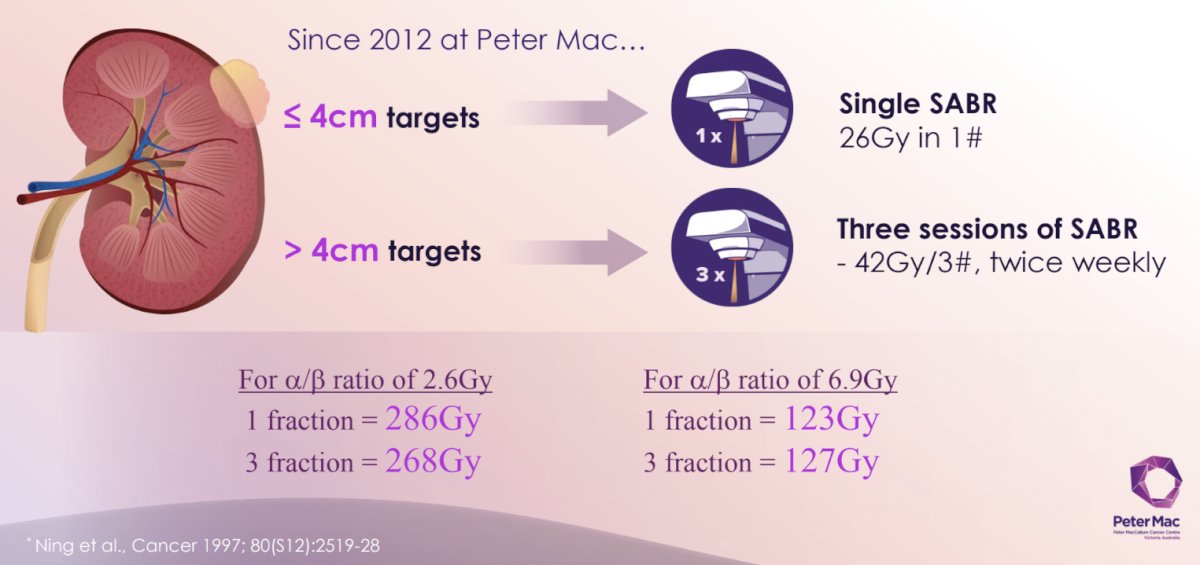
In answer to the question of the optimal dose regimen for SABR, Dr. Siva stated the following:
- Single-fraction SABR 25–26 Gy appears to be a favorable regimen
- A dose of 42–48 Gy in three fractions is reasonable and is used in prospective trials
- If a 5-fraction regimen is used due to difficulty meeting OAR constraints, 40 Gy in 5 fractions could be reasonable
What is the role of post-treatment kidney biopsy? He argued that post-radiotherapy biopsy or surgical dissection has been discarded in the treatment paradigm of many other malignancies treated with radical radiotherapy. So why do we need it post-SABR?
Studies of post-SABR kidney biopsies have demonstrated that SABR decreases cellularity, the Ki67 index (marker of cellular proliferation), and transcriptomically engages senescence and apoptotic pathways.6 There also appears to be a discord between local control rates and biopsy ‘positivity’, with Tang et al. demonstrating that almost 60% of patients had evidence of a positive biopsy at 3 months post-SABR, but only 8% had a documented local failure.7 As such, Dr. Siva noted the following regarding the role of post-SABR biopsies:
- Given that a positive biopsy does not predict subsequent local tumour and distant progression, it is only recommended when imaging findings are concerning for progression.
- Do not biopsy following SABR outside the context of a clinical trial
- In contrast, pre-treatment biopsy is recommended
What about SABR for patients with solitary kidneys? From an efficacy standpoint, there do not appear to be any differences in local failure rates, cancer-specific survival, or progression-free survival among patients with solitary versus dual kidneys receiving SABR.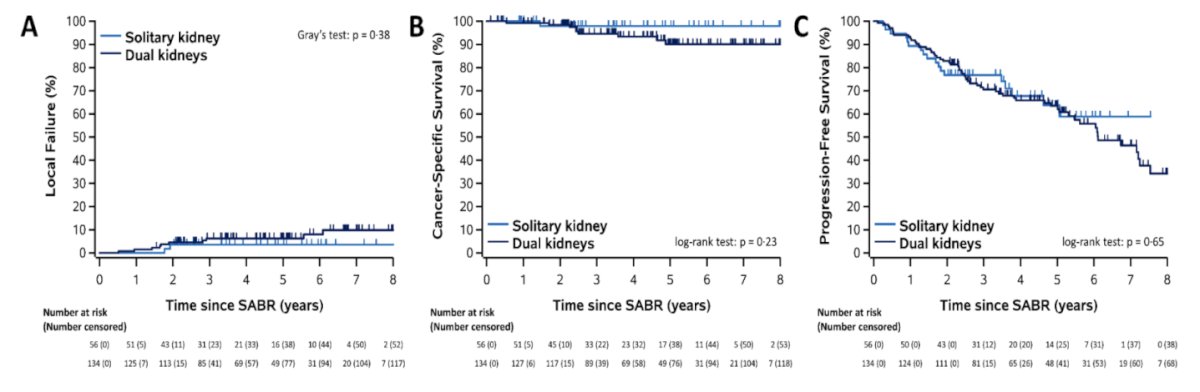
At 5 years post-SABR, patients with solitary kidneys had a 14.5 mL/min mean decrease in eGFR, compared to a 13.3 mL/min mean decrease in patients with dual kidneys (p=0.67). There were similar rates of post-SABR dialysis in both groups (3.6–3.7%).5,8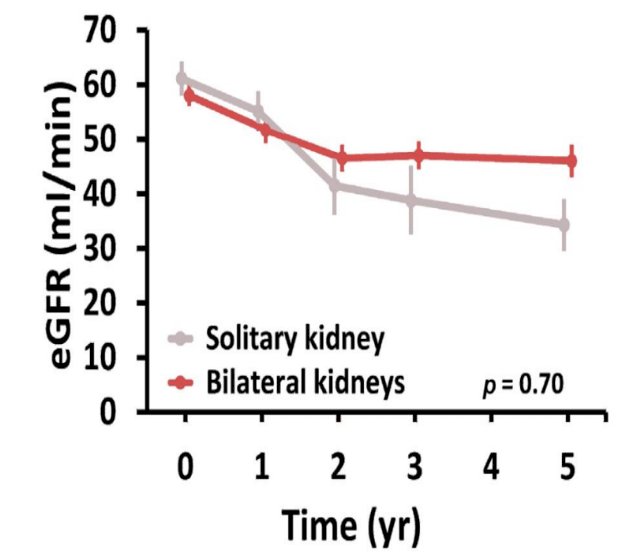
There appears to be a dose-response relationship between the SABR dose administered and the eGFR decrease.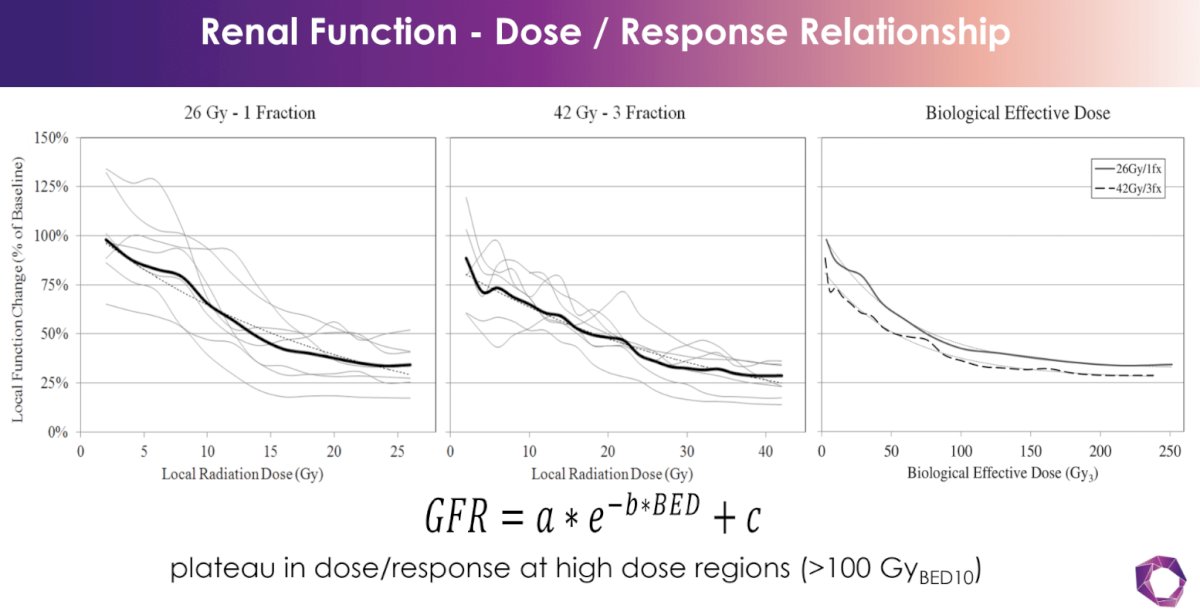
Dr. Siva noted the following with regards to SABR and renal function:
- Patient and treatment factors need to be considered. Refer patients to a renal physician to manage medical CKD.
- Caution is advised in CKD class IV-V patients. Shared decision-making is key due to the risk of dialysis.
- Technical considerations include reducing the volume of irradiated kidney, particularly for intermediate dose wash (50% conformality).
What is the optimal post-treatment follow-up schedule? Summarized below is the schedule of assessments over 5 years, adapted from FASTRACK II.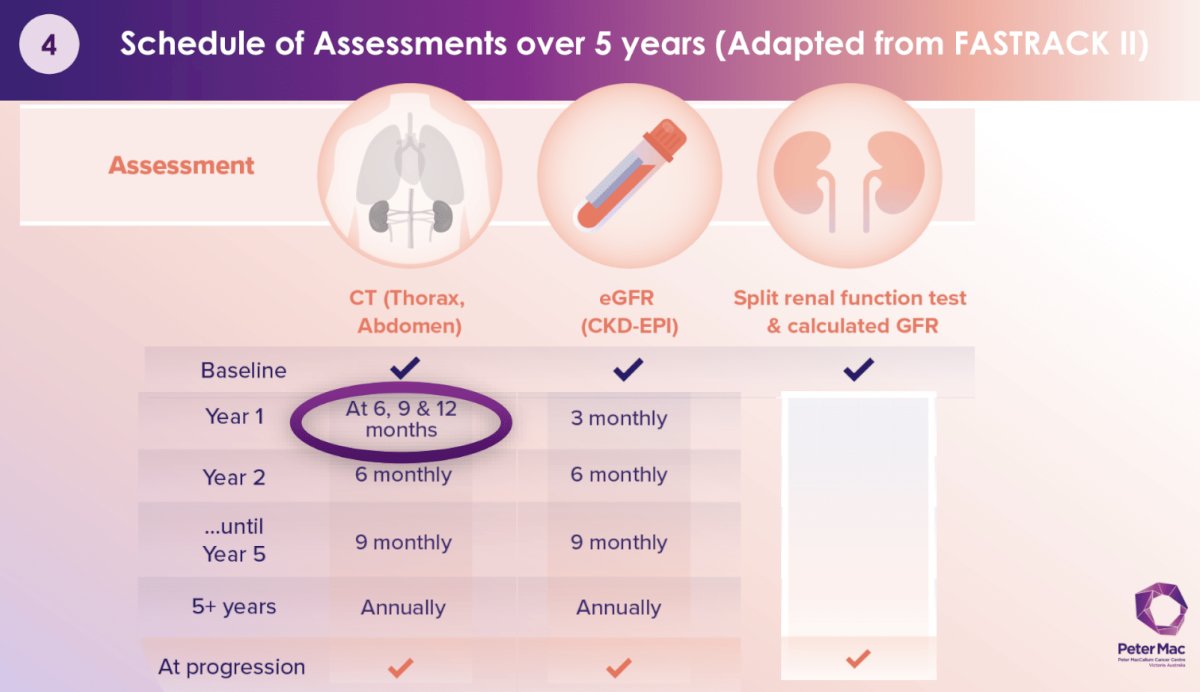
For serial tumor size evaluation, Dr. Siva noted that we can expect an ongoing tumor size reduction/response over numerous years as demonstrated by Funayama et al. in 2019.9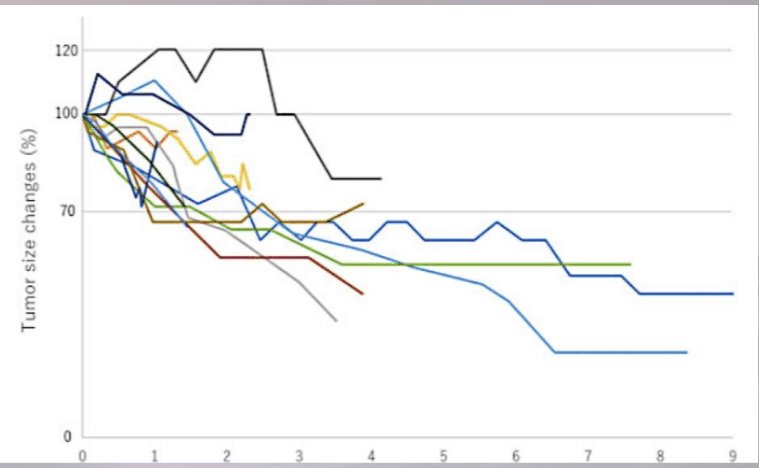
To date, it appears that we cannot rely on CT contrast enhancement to discern responders from non-responders. In a study of 41 RCC patients receiving SABR, 93% of patients had evidence of local control, despite having higher post-treatment enhancement on CT, compared to the pre-treatment setting.10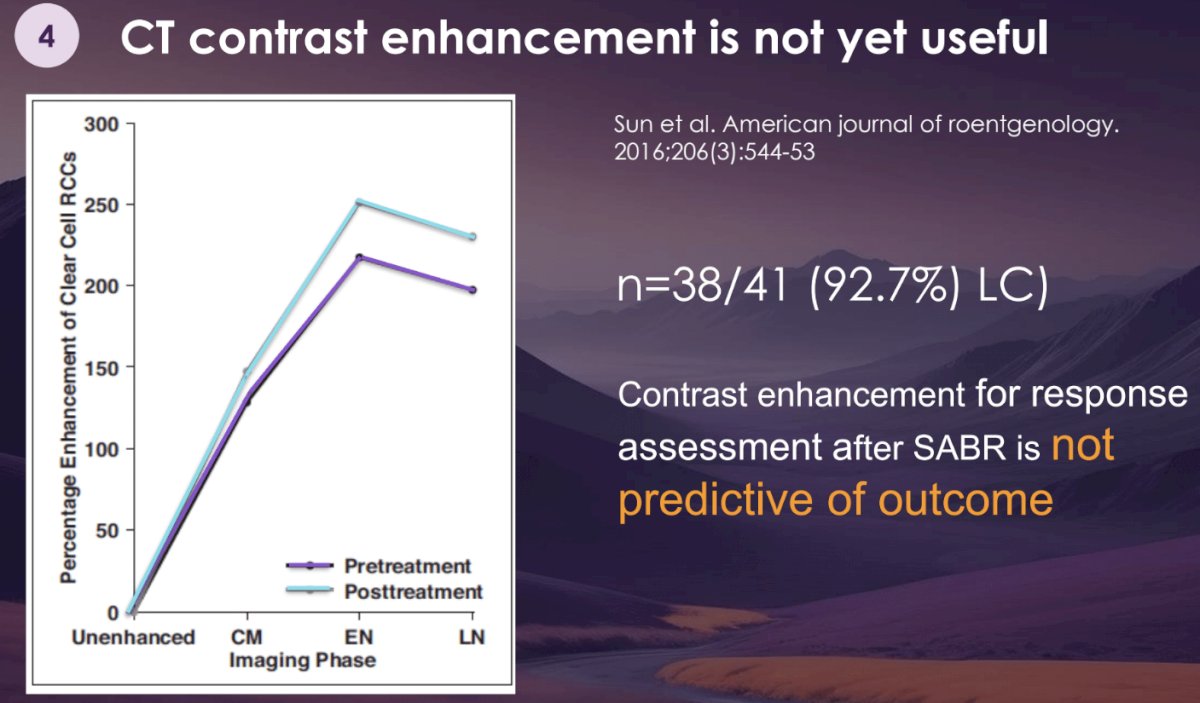
For post-treatment follow-up, Dr. Siva noted the following:
- RECIST measurement is still standard but remains imperfect
- At 3 months post-SABR, pseudoprogression is often noted. Avoid CT imaging until ~6 months post-treatment.
- Contrast enhancement is not recommended for response evaluation (in contrast to thermal ablation).
Dr. Siva concluded by noting that the future may include a comparative randomized trial of SABR versus surgery; but, for the time being, SABR for the treatment of primary RCC is ready for ’primetime’.
Presented by: Shankar Siva, PhD, MBBS, FRANZCR, Radiation Oncologist, Professor of Medicine, Peter MacCallum Cancer Centre, University of Melbourne, Melbourne, Australia
Written by: Rashid Sayyid, MD, MSc – Robotic Urologic Oncology Fellow at The University of Southern California, @rksayyid on Twitter during the 2024 ASTRO Annual Congress held in Washington, DC between September 29th and October 2nd, 2024
- Hsieh JJ, Purdue MP, Signoretti S, et al. Renal cell carcinoma. Nat Rev Dis Primers. 2017; 3:17009.
- Tunio MA, Hashmi A, Rafi M. Need for a new trial to evaluate postoperative radiotherapy in renal cell carcinoma: a meta-analysis of randomized controlled trials. Ann Oncol. 2010; 21(9):1839-45.
- Ning S, Trisler K, Wessels BW, Knox SJ. Radiobiologic studies of radioimmunotherapy and external beam radiotherapy in vitro and in vivo in human renal cell carcinoma xenografts. Cancer. 1997; 80(12 Suppl):2519-28.
- Siva S, Bressel M, Sidhom M, et al. Stereotactic ablative body radiotherapy for primary kidney cancer (TROG 15.03 FASTRACK II): a non-randomized phase 2 trial. Lancet Oncol. 2024; 25(3):308-16.
- Siva S, Ali M, Correa RJM, et al. 5-year outcomes after stereotactic ablative body radiotherapy for primary renal cell carcinoma: an individual patient data meta-analysis from IROCK (the International Radiosurgery Consortium of the Kidney). Lancet Oncol. 2022; 23(12):1508-16.
- Hannan R, McLaughlin MF, Pop LM, et al. Phase 2 Trial of Stereotactic Ablative Radiotherapy for Patients with Primary Renal Cancer. Eur Urol. 2023; 84(3): 275-86
- Tang C, Msaouel P, Hara K, et al. Definitive radiotherapy in lieu of systemic therapy for oligometastatic renal cell carcinoma: a single-arm, single-center, feasibility, phase 2 trial. Lancet Oncol. 2021; 22(12):1732-9.
- Tan VS, Correa RJM, Warner A, et al. Long-term Renal Function Outcomes After Stereotactic Ablative Body Radiotherapy for Primary Renal Cell Carcinoma Including Patients with a Solitary Kidney: A Report from the International Radiosurgery Oncology Consortium of the Kidney. Eur Urol Oncol. 2024.
- Funayama S, Onishi H, Kuriyama K, et al. Renal Cancer is Not Radioresistant: Slowly but Continuing Shrinkage of the Tumor After Stereotactic Body Radiation Therapy. Technol Cancer Res Treat. 2019; 18:1533033818822329.
- Sun MRM, Brook A, Powell MF, et al. Effect of Stereotactic Body Radiotherapy on the Growth Kinetics and Enhancement Pattern of Primary Renal Tumors. AJR Am J Roentgenol. 2016; 206(3):544-53.


