(UroToday.com) The 2024 American Society for Radiation Oncology (ASTRO) Annual Meeting held in Washington, DC between September 29th and October 2nd, 2024, was host to a presidential symposium of innovations in genitourinary cancers, specifically addressing the ‘renaissance’ of radiotherapy for renal cell carcinoma (RCC). Dr. Chad Tang discussed the strategies and evidence for stereotactic ablative radiotherapy (SABR) in RCC, focusing on metastasis-directed therapy (MDT) in lieu of systemic therapy and MDT for oligoprogressive disease.
The first report of MDT for the treatment of oligometastatic RCC was published in 1938 when a patient with kidney cancer underwent a nephrectomy followed by a sub-total lobectomy for a pulmonary metastasis 10 months later and has since demonstrated long-term survival.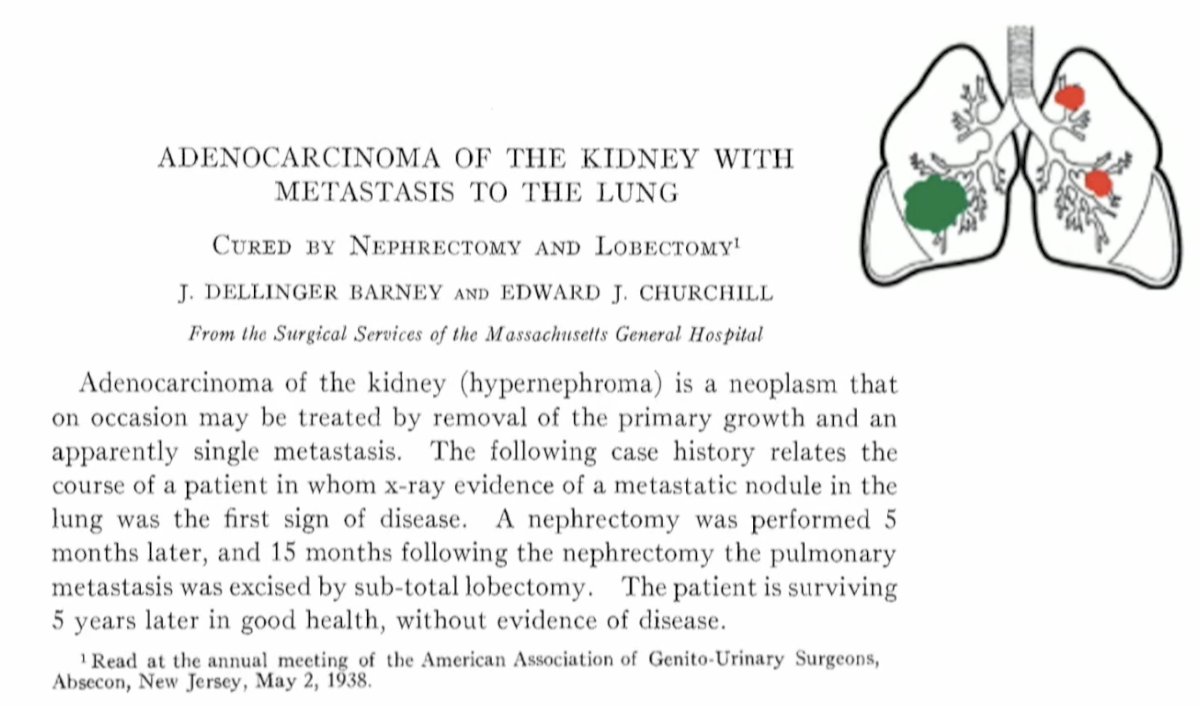
In 2016, Rini et al. published the results of a prospective phase II trial of 48 treatment-naïve, asymptomatic, metastatic RCC patients who underwent active surveillance for their metastatic disease. Patients continued on observation until initiation of systemic therapy for metastatic RCC – a decision that was made at the discretion of the treating physician and patient. The median time on surveillance was 14.9 months, and the median progression-free survival was 9.4 months. Notably, although none had received systemic therapy prior to entry, 7/48 patients had received MDT while on trial.1 Dr. Tang noted that this was early evidence that MDT may have efficacy in this setting.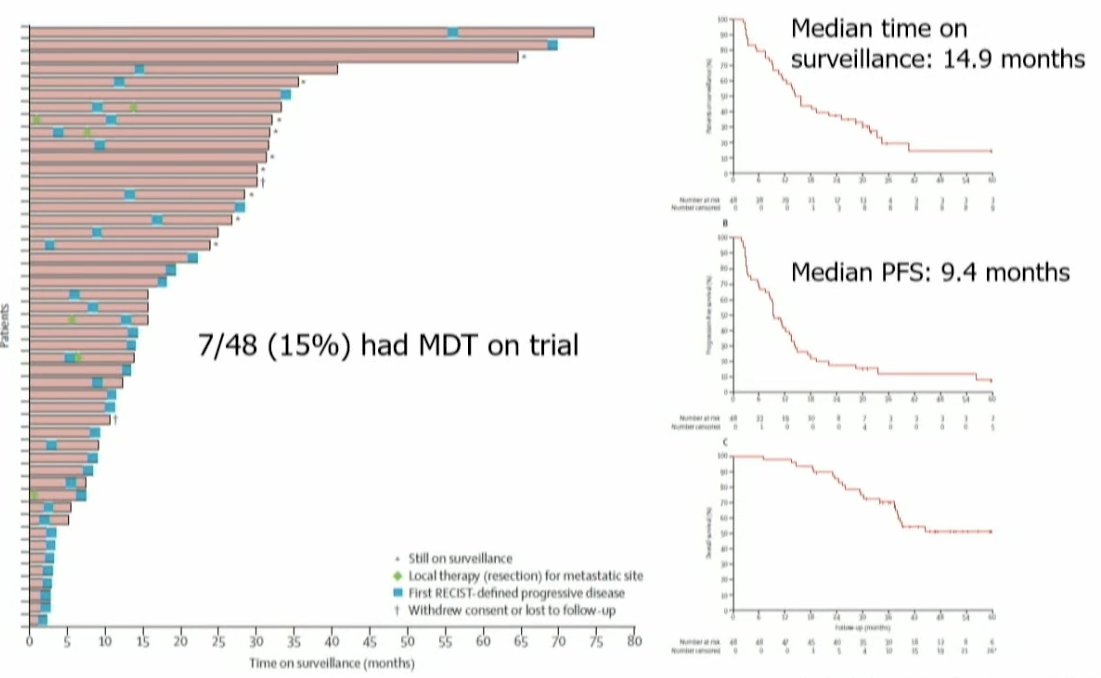
In 2021, Dr. Tang and colleagues published the results of a single-arm phase II feasibility trial that evaluated the efficacy of MDT for RCC patients. Eligible patients were those with ≤5 metastatic lesions and had received no more than one prior line of systemic therapy (not within the month prior to enrollment). Patients were treated with stereotactic body radiotherapy (defined as ≤5 fractions with ≥7 Gy per fraction) to all lesions and maintained off systemic therapy. When lesion location precluded safe SABR administration, patients were treated with hypofractionated intensity-modulated radiotherapy regimens (60–70 Gy in 10 fractions or 52.5–67.5 Gy in 15 fractions). If patients experienced progression, then systemic therapy was initiated only if there was evidence of polymetastatic progression (>3 sites of disease).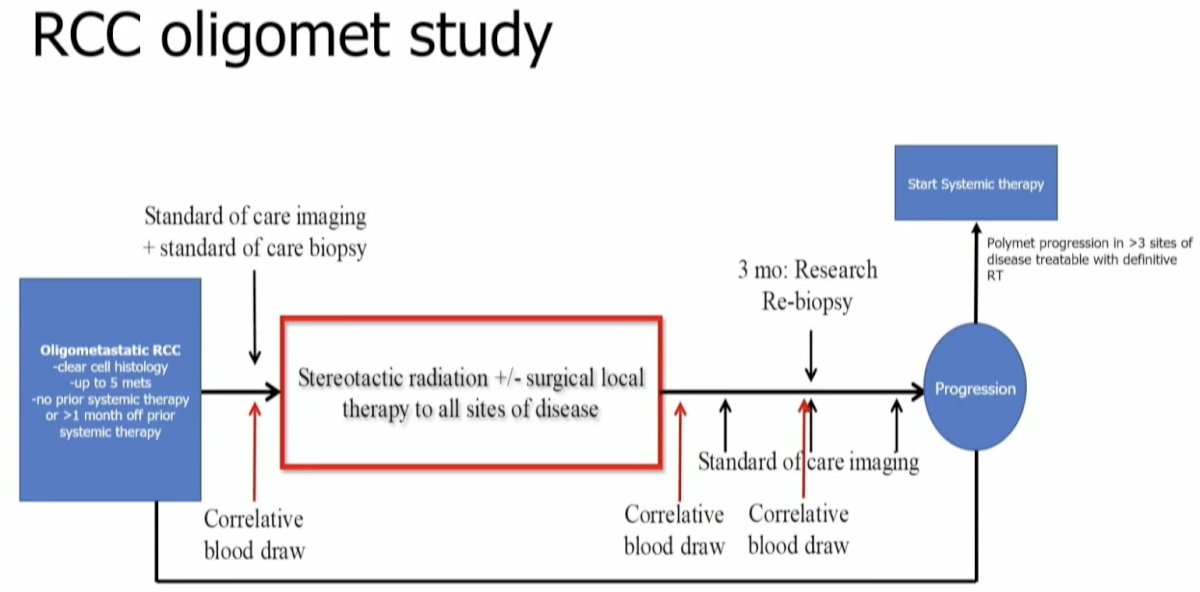
30 patients were enrolled between July 2018 and September 2020. All patients had clear cell histology and had undergone a nephrectomy prior to enrolment. All patients completed ≥1 round of radiotherapy with <7 days of unplanned breaks. At a median follow-up of 17.5 months, the median progression-free survival was 22.7 months, with a 1-year progression-free survival of 64%. Many trial participants have undergone numerous rounds of SABR (up to 7 in some patients). Three (10%) patients had severe adverse events: two grade 3 (back pain and muscle weakness) and one grade 4 (hyperglycemia) adverse events were observed. There were no treatment-related deaths.2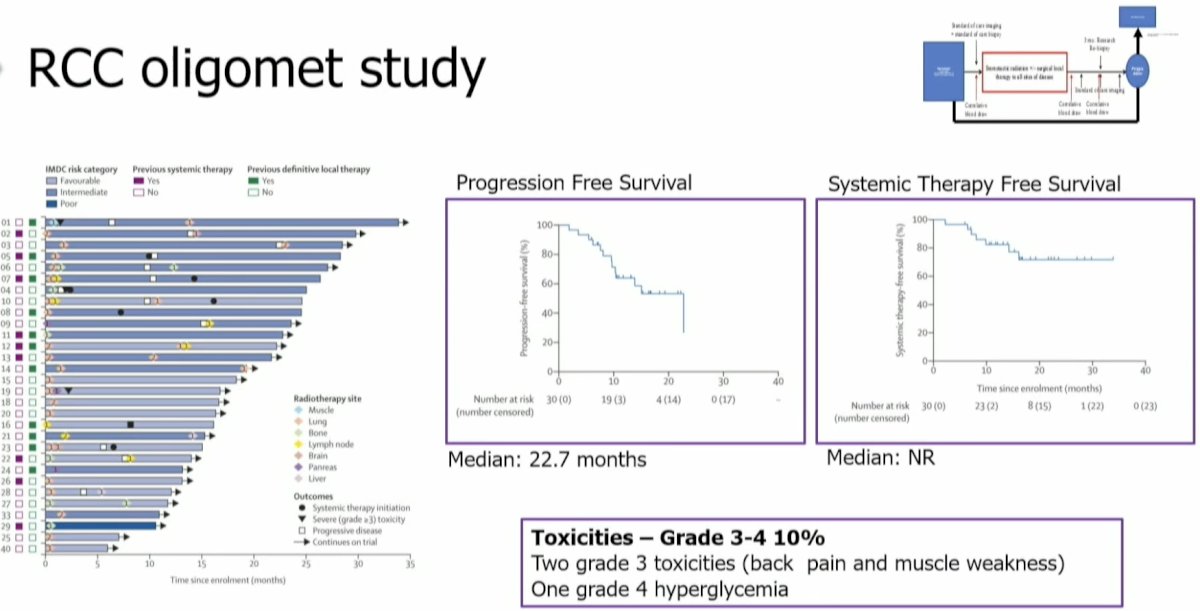
Concurrently, the group from UT Southwestern, led by Dr. Hannan, published the results of an analogous phase II trial that demonstrated similar results, with a 1-year progression-free survival of 83%, a 1-year systemic therapy-free survival of 91%, and grade ≥3 toxicity rate of 4%.3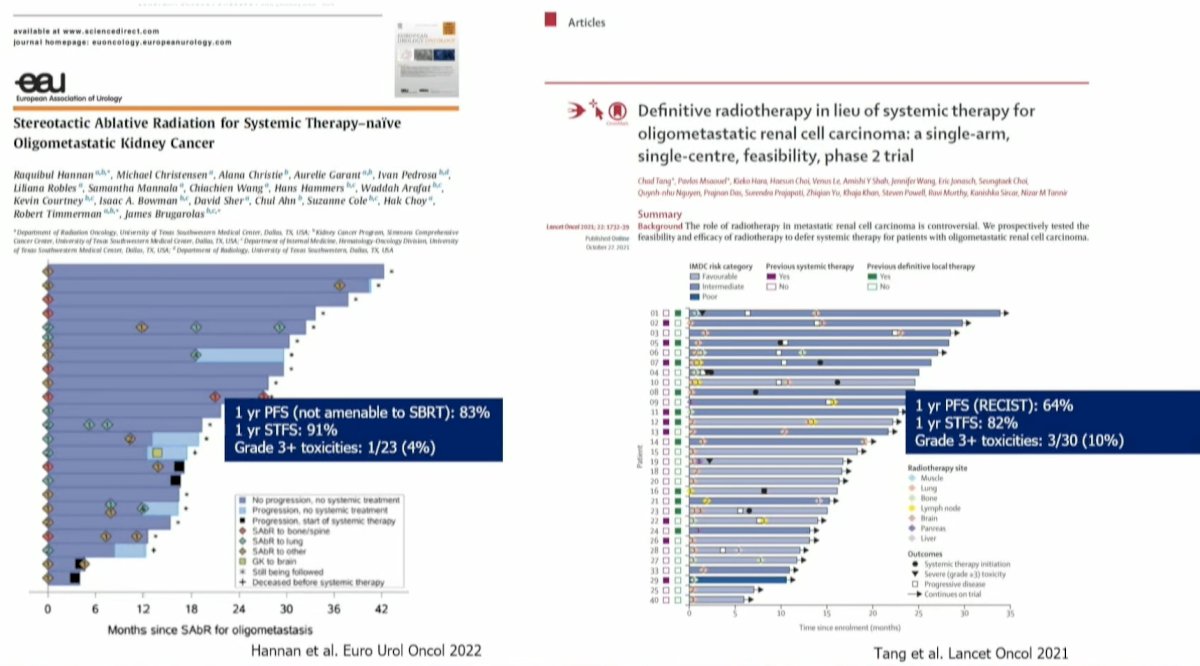
The EA8211/SOAR trial is an ongoing non-inferiority, phase III, randomized trial of oligometastatic RCC patients who are being randomized to either SABR alone (systemic therapy at progression) versus systemic therapy alone, with a primary endpoint of overall survival.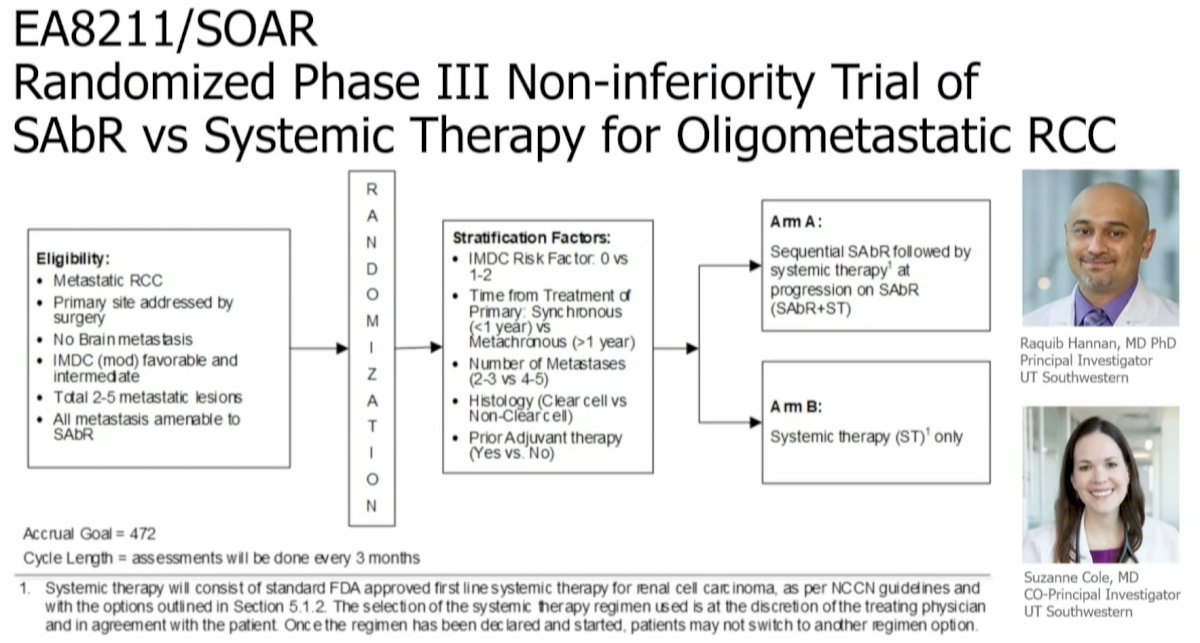
Why design this trial as a non-inferiority trial? In other words, why would SABR being non-inferior to systemic therapy be considered a success? The use of doublet immunotherapy systemic regimens is significantly more expensive than SABR ($150-300K/annually versus $20-50K/annually for SABR). SABR generally has a more favorable toxicity profile and requires less frequent healthcare visits.4
Further evidence to support MDT comes from the recently published KEYNOTE-564 trial of adjuvant pembrolizumab. In this trial, high-risk RCC patients who underwent a radical or partial nephrectomy were randomized to one year of pembrolizumab 200 mg IV every three weeks versus placebo.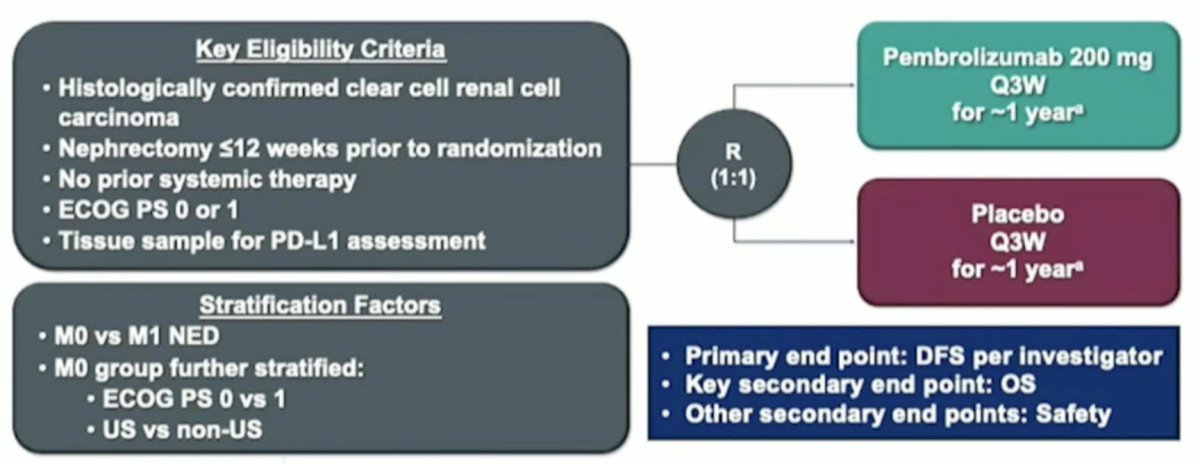
Adjuvant pembrolizumab significantly improved overall survival in the overall cohort (HR: 0.62, 95% CI: 0.44–0.87, p=0.005). On subgroup analysis, the highest survival benefit observed was in the M1 NED subgroup (i.e., those who were metastatic but had resection of their disease), which is a subgroup that received ‘upfront’ MDT.5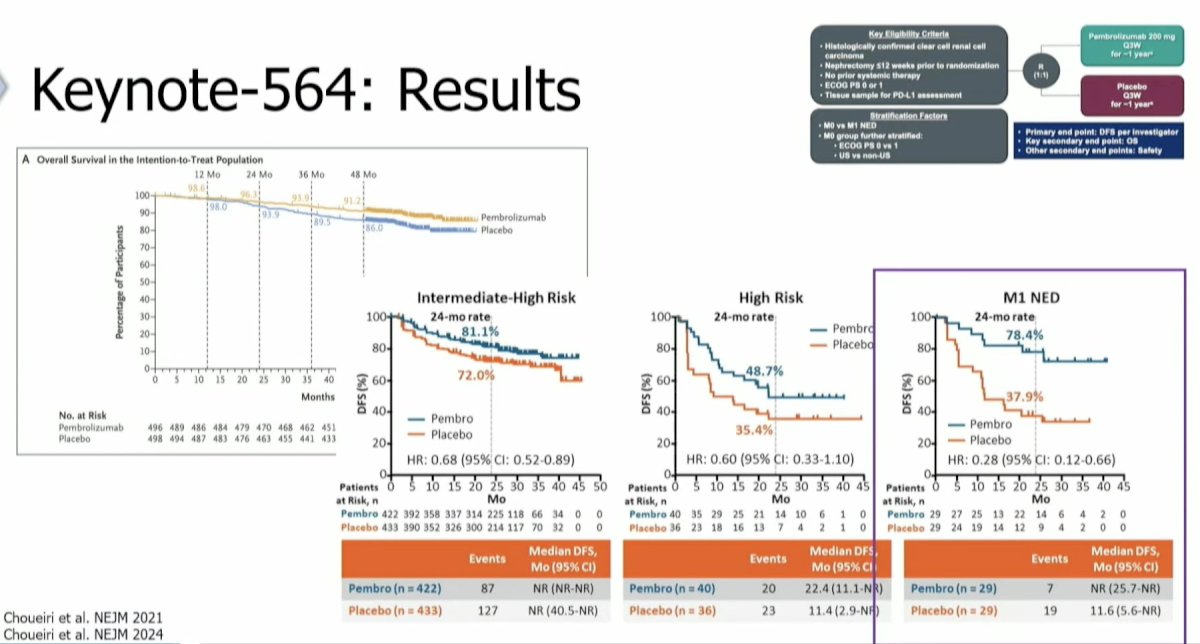
The sequential combination of MDT followed by pembrolizumab was evaluated in the phase II RAPPORT trial by Siva et al.6 This trial included 30 patients with oligometastatic clear cell RCC (1–5 lesions) who received SABR (20 Gy in 1 fraction) or palliative radiotherapy (30 Gy in 10 fractions) followed by 6 months of pembrolizumab. The median progression-free survival was 16 months, and the adverse event profile was favorable overall (grade 3: 13%). 63% of patients experienced either a complete or partial response.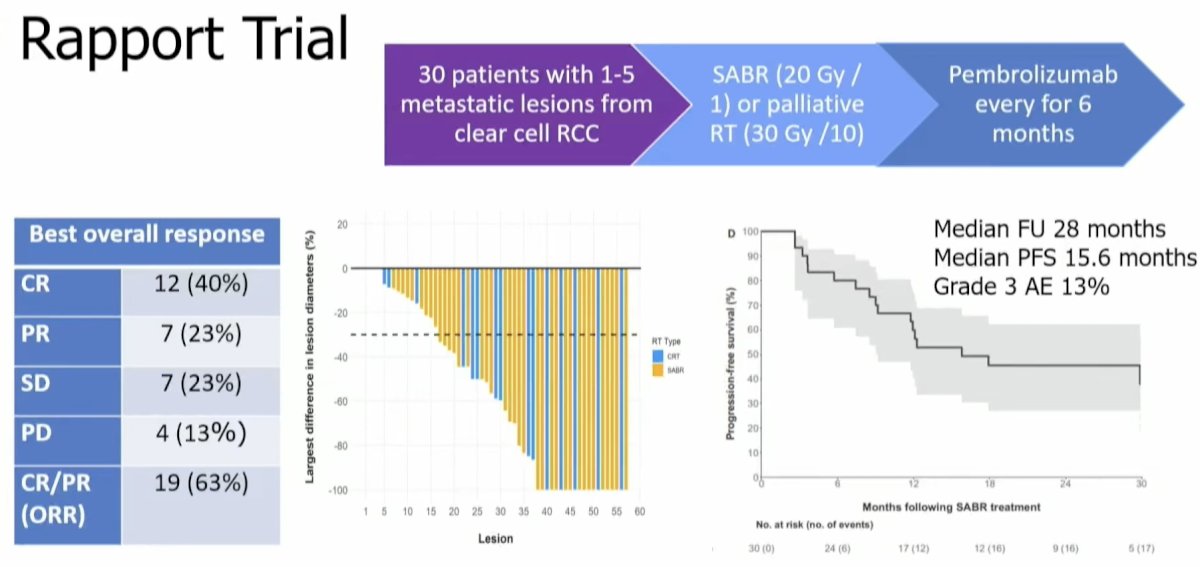
Based on these results and additional evidence in this space, Dr. Tang and colleagues have initiated a randomized phase II trial whereby oligometastatic RCC patients will receive radiotherapy to all sites of visible disease followed by pembrolizumab for 1 year versus observation. At the time of oligometastatic progression, patients will crossover to the other arm (i.e., observation to pembrolizumab and vice versa). At polymetastatic progression, patients would come off trial. The primary endpoint is progression-free survival.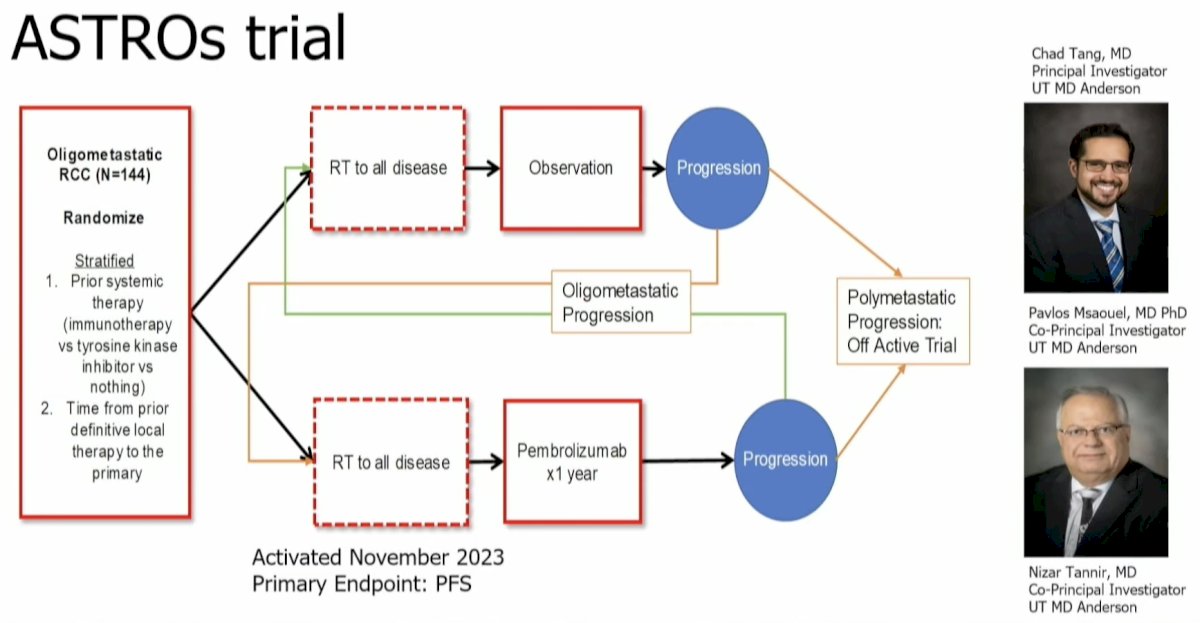
What about MDT for oligoprogressive disease? This refers to a population of patients with an unlimited number of metastatic lesions initially, but despite active systemic therapy, have a limited number of growing lesions (majority are controlled).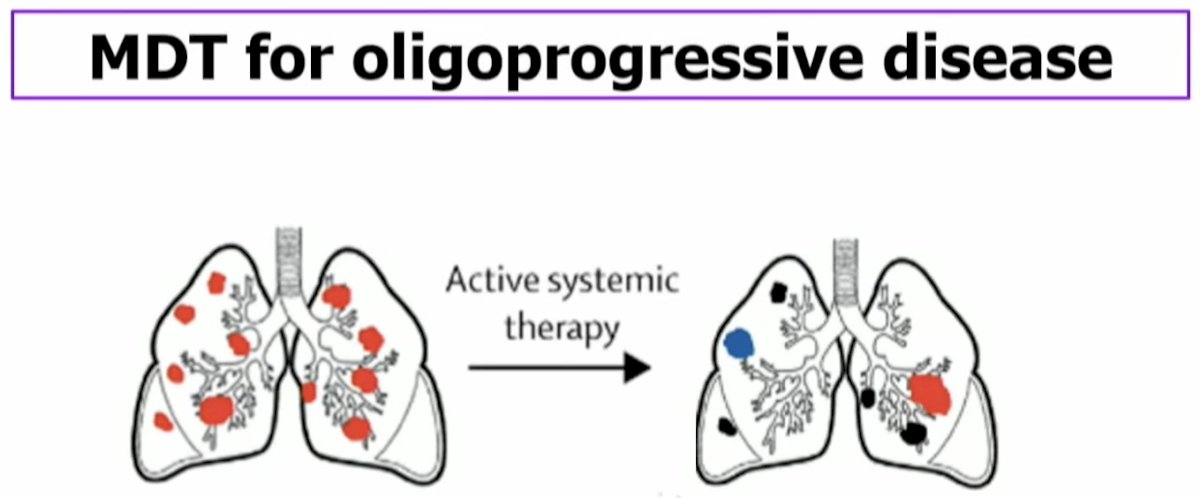
To date, there have been two trials that have evaluated the role of MDT in this setting. In these two trials (Cheung et al., European Urology in 2021; Hannan et al., European Urology Oncology in 2022), all oligo progressive RCC patients received SABR to all sites of progressive disease, while systemic therapy was continued.7,8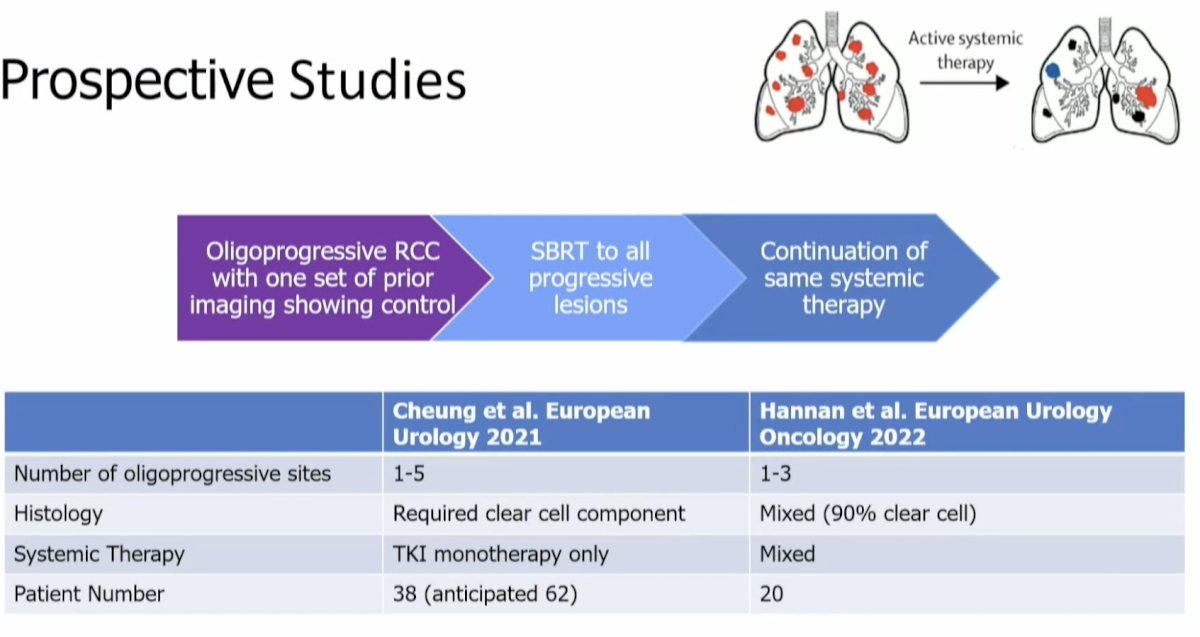
These two trials demonstrated similar results. The median progression-free survival was ~1 year, and the grade ≥3 toxicity rate was ≤5%.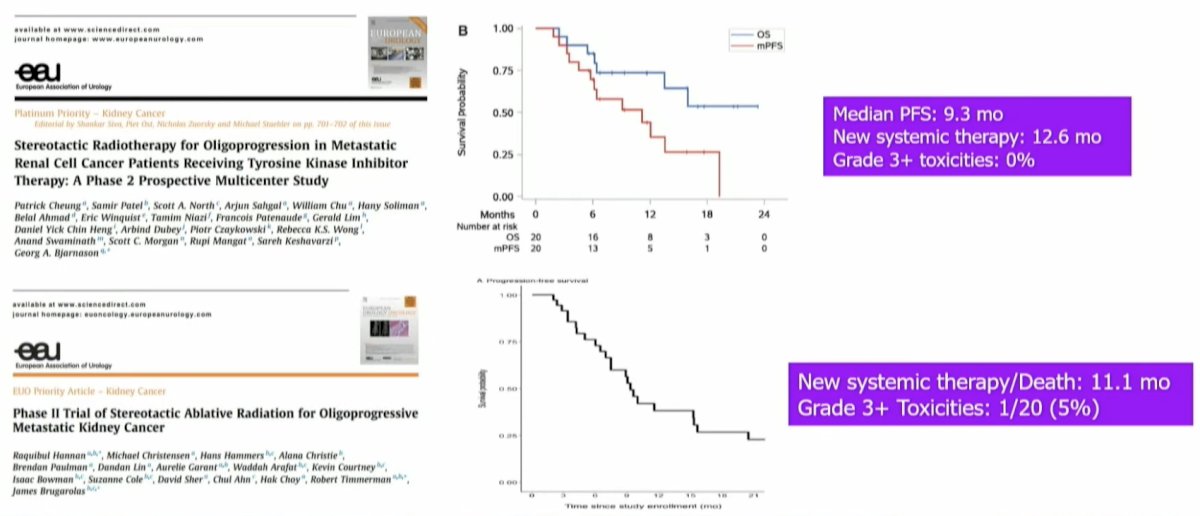
The EXTEND-OP trial is a basket trial (including prostate and RCC patients) that is randomizing oligoprogressive patients to next-line systemic therapy versus local consolidative therapy to all oligoprogressive sites and continued same-line systemic therapy. At the time of progression in the experimental arm, patients would receive next-line systemic therapy.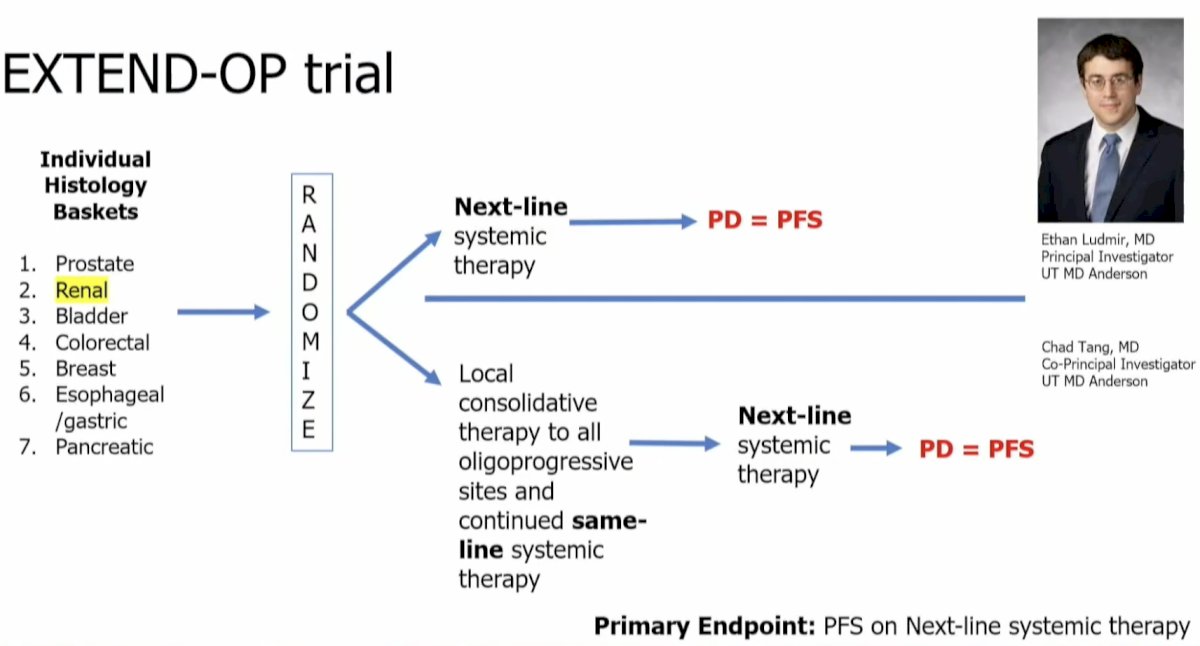
What about SABR for the treatment of primary RCC lesions? As Dr. Siva had previously addressed this topic extensively in the preceding presentation, Dr. Siva did not do a ‘deep dive’ into this topic. He did note, however, that similar to the cytoreductive nephrectomy approach evaluated in the SWOG and EORTC studies (interferon era) and CARMENA trial (TKI era), SABR to the primary RCC lesion in the metastatic setting is being evaluated in CYTOSHRINK (NCT04090710), which is randomizing patients with untreated, IMDC intermediate/poor risk RCC 2:1 to the experiment arm of SABR to the primary RCC lesion + ipilimumab/nivolumab versus ipilimumab/nivolumab alone.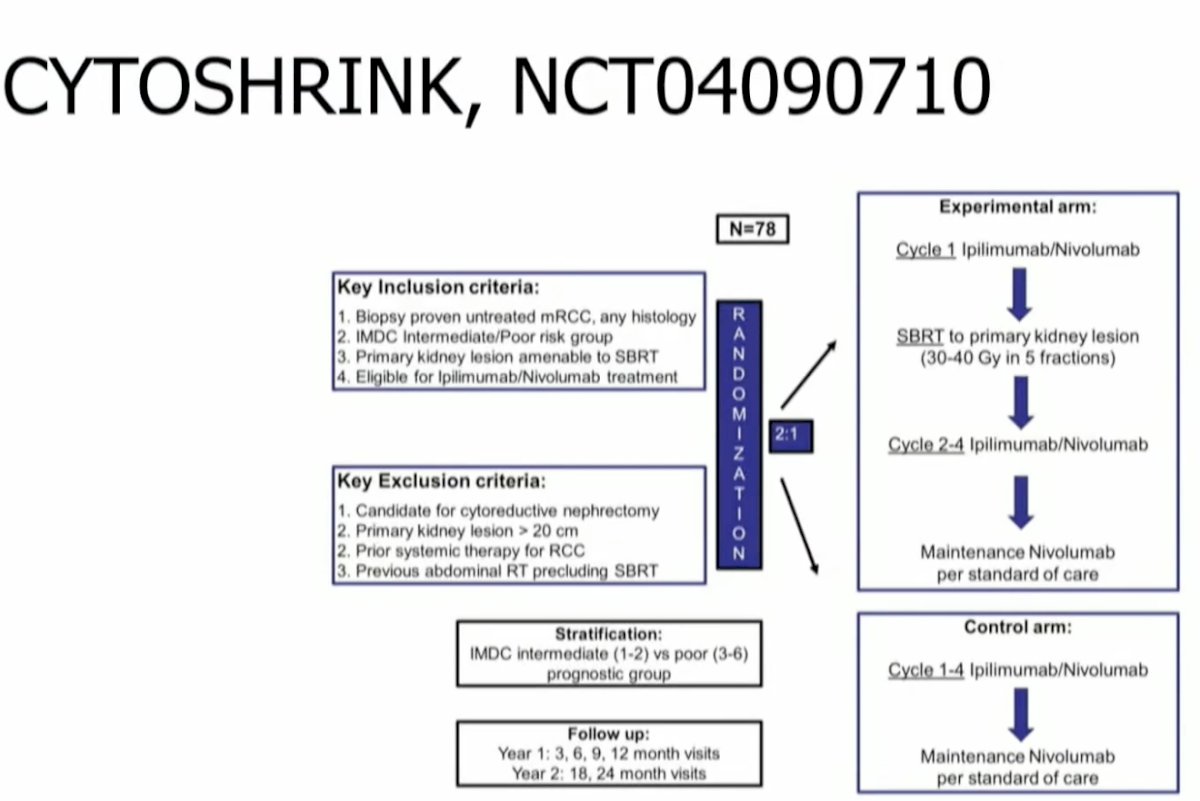
NRG GU-012 (NCT05327686) is randomizing IMDC intermediate/poor risk RCC patients to standard immunotherapy +/- SABR to the primary: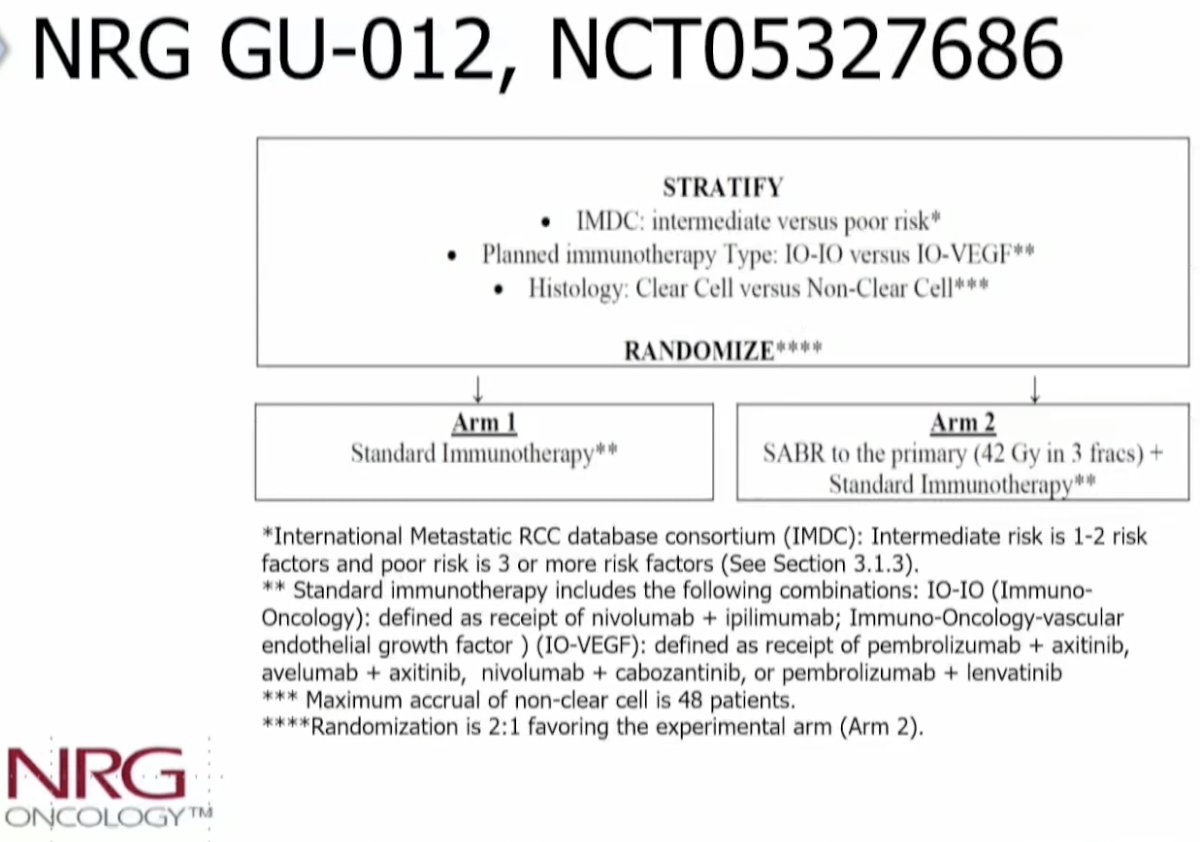
The ITALIC-RCC trial uniquely randomizes patients into a multiarm trial based on the primary tumor lesion size. Patients with tumors ≤4 cm are being randomized into one of three arms: Anti-PD-1 based therapy +/- radiotherapy or +/- surgery. Patients with primary tumors >4 cm will be randomized to anti-PD-1-based therapy +/- surgery.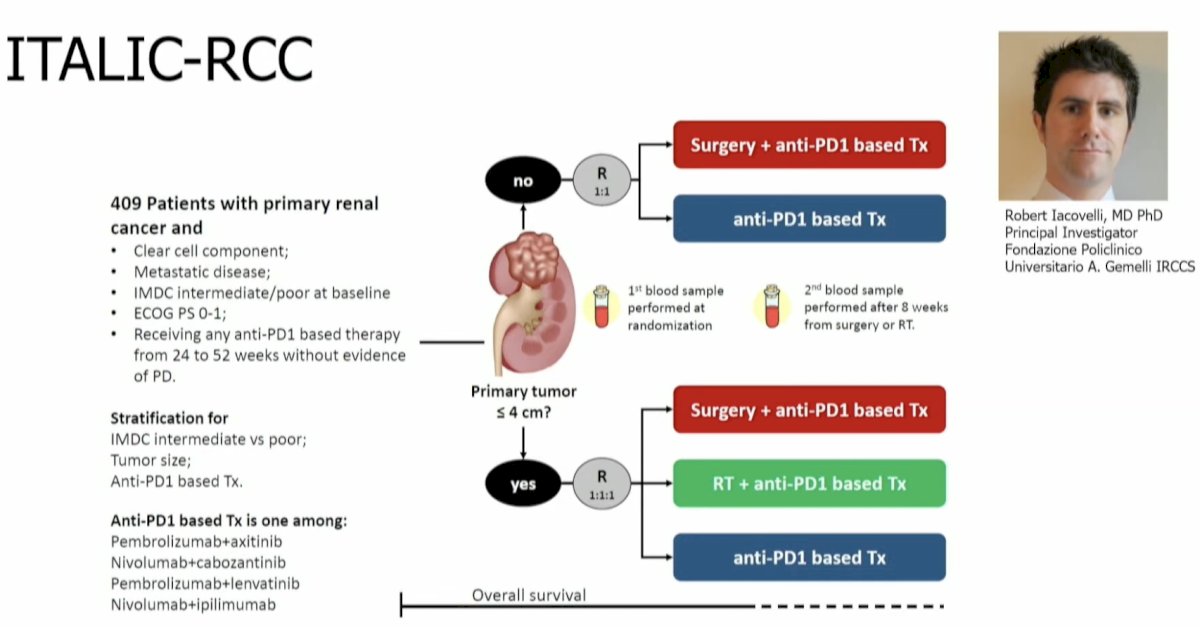
Another future direction is SABR for IVC tumor thrombi. Thrombi of the IVC occur in 4–10% of advanced RCC cases and can produce edema and hepatic congestion. The standard of care is extirpative surgery, but that can be a complex/morbid procedure. In a multinational cases series of 15 patients treated with SABR, it was demonstrated that all patients experienced symptom palliation and 58% achieved a radiographic response. MRI-based radiation can improve the precision with onbosetupt up and facilitate motion management.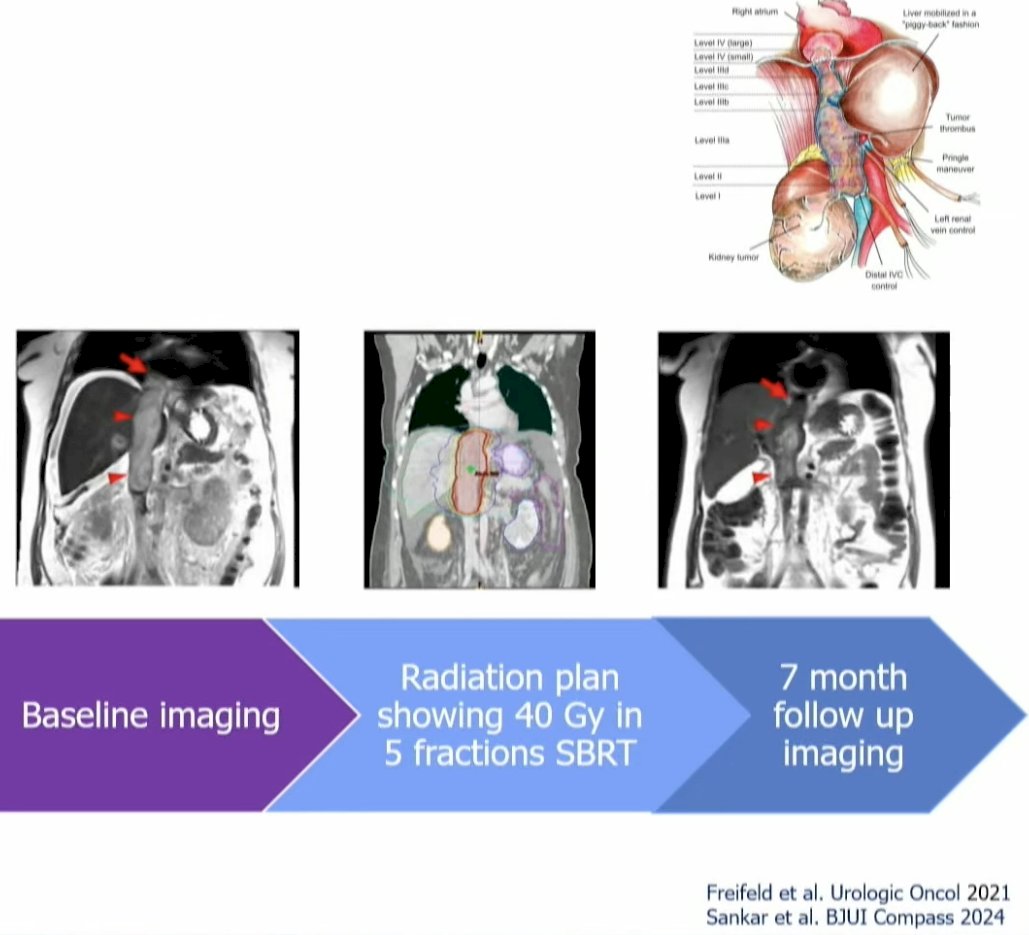
What about SABR for variant histology RCC? Dr. Tang focused on its potential role in renal medullary carcinoma. This is a rare and almost universally fatal disease affecting predominantly young African American men and women. It predominantly occurs in patients with sickle cell trait disease or anemia due to sickling in the medulla of the kidney during times of hypoxia. These tumors are prone to replicative stress and are treated with chemotherapy instead of targeted therapy.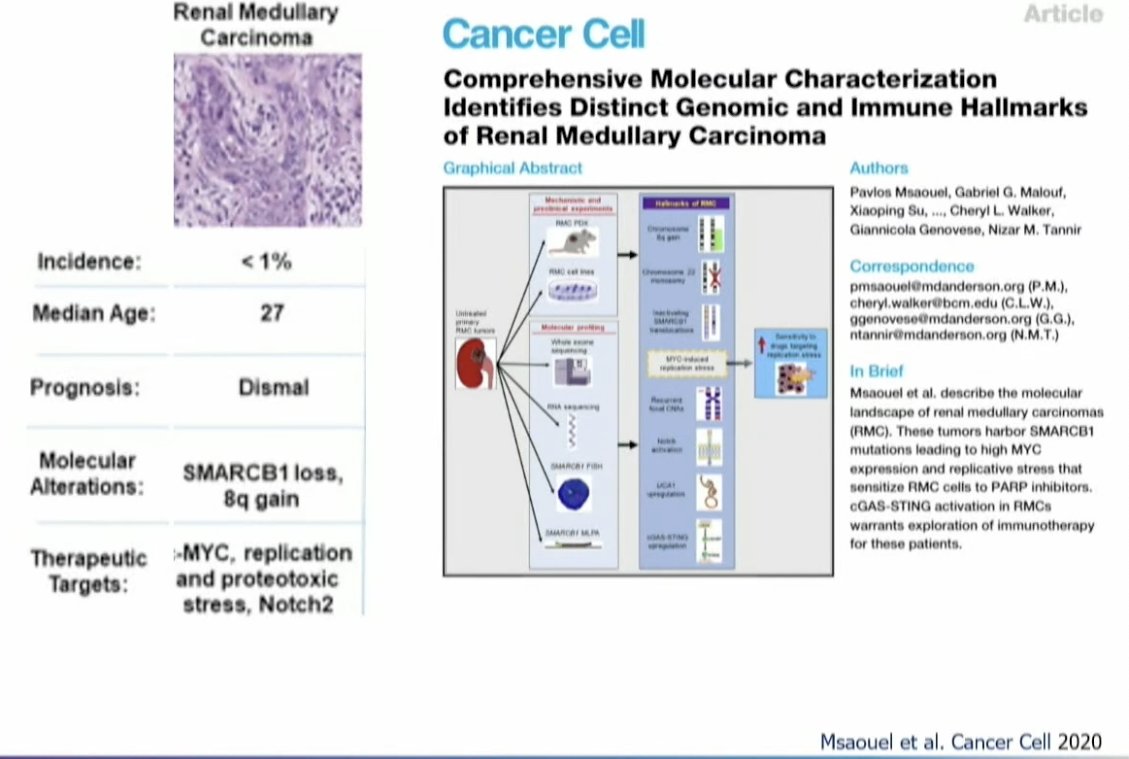
Recently Dr. Tang and colleagues published the results of a case series of 5 renal medullary carcinoma patients who had progressed on 1–3 lines of systemic therapy and received SABR to all sites of visible disease. Overall, 3/5 patients were rendered ‘NED’ and stopped systemic therapy without evidence of recurrence to date.9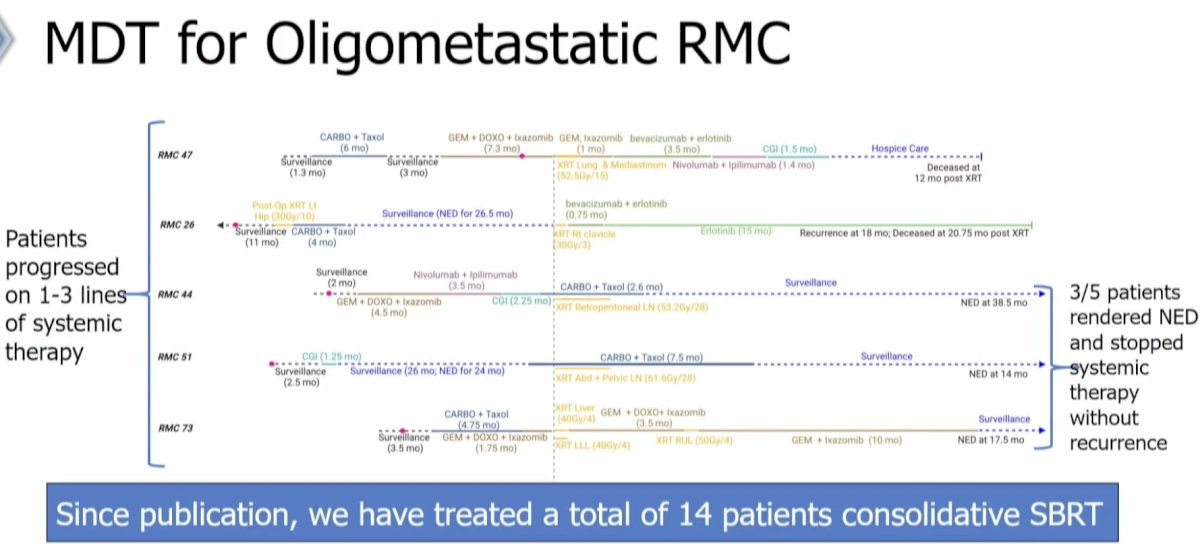
Dr. Tang concluded his presentation by highlighting once again the current and potential applications of SABR for the treatment of advanced RCC patients: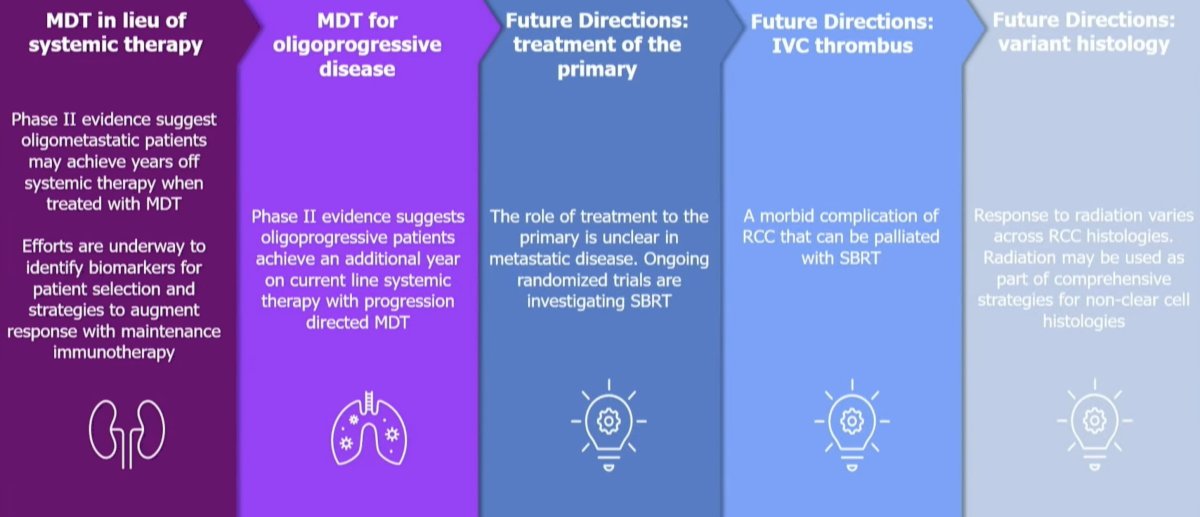
Presented by: Chad Tang, MD, MS, Associate Professor, Department of Radiation Oncology, Division of Radiation Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX
Written by: Rashid Sayyid, MD, MSc – Robotic Urologic Oncology Fellow at The University of Southern California, @rksayyid on Twitter during the 2024 ASTRO Annual Congress held in Washington, DC between September 29th and October 2nd, 2024
References:- Rini BI, Dorff TB, Elson P, et al. Active surveillance in metastatic renal-cell carcinoma: a prospective, phase 2 trial. Lancet Oncol. 2016; 17(9):1317-24.
- Tang C, Msaouel P, Hara K, et al. Definitive radiotherapy in lieu of systemic therapy for oligometastatic renal cell carcinoma: a single-arm, single-center, feasibility, phase 2 trial. Lancet Oncol. 2021; 22(12):1732-9.
- Hannan R, McLaughlin MF, Pop LM, et al. Phase 2 Trial of Stereotactic Ablative Radiotherapy for Patients with Primary Renal Cancer. Eur Urol. 2023; 84(3):275-86.
- Tang C, Msaouel P. Charting the Path to Systemic Therapy De-escalation-Oligometastatic Kidney Cancer as a Paradigm. JAMA Oncol. 2024; 10(5):561-2.
- Choueiri TK, Tomczak P, Park SG, et al. Overall Survival with Adjuvant Pembrolizumab in Renal-Cell Carcinoma. N Engl J Med. 2024; 390:1359-71.
- Siva S, Bressel M, Wood ST, et al. Stereotactic Radiotherapy and Short-course Pembrolizumab for Oligometastatic Renal Cell Carcinoma-The RAPPORT Trial. Eur Urol. 2022; 81(4):364-72.
- Cheung P, Patel S, North SA, et al. Stereotactic Radiotherapy for Oligoprogression in Metastatic Renal Cell Cancer Patients Receiving Tyrosine Kinase Inhibitor Therapy: A Phase 2 Prospective Multicenter Study. Eur Urol. 2021; 80(6):693-700.
- Hannan R, Christensen M, Hammers H, et al. Phase II Trial of Stereotactic Ablative Radiation for Oligoprogressive Metastatic Kidney Cancer. Eur Urol Oncol. 2022; 5(2):216-24.
- Mbilnyi RH, Msaouel P, Rao P, et al. Radiation Therapy for the Management of Renal Medullary Carcinoma: A Multi-Case Study. Clin Genitourin Cancer. 2024; 22(3):102065.


