(UroToday.com) The 2024 American Society for Radiation Oncology (ASTRO) annual meeting held in Washington D.C., between September 29 and October 2 was host to the session GU Quick Pitch (GU 4). Dr. Omar Azem discussed the impact of prior local therapy on treatment response and survival of men with metastatic castrate resistant prostate cancer (mCRPC) in a secondary analysis of the COU-AA-302 and ACIS Trials.
In vitro studies have indicated that definitive local therapy (LT), such as radiation therapy (RT) or radical prostatectomy (RP) for prostate cancer, can induce cellular changes, including neuroendocrine differentiation. These changes can potentially lead to resistance to subsequent systemic therapies, including androgen receptor pathway inhibitors (ARPIs).1 However, these findings have not been conclusively translated into clinically meaningful differences in treatment effects, particularly in patients with metastatic castration-resistant prostate cancer (mCRPC) and metastatic hormone-sensitive prostate cancer (mHSPC).2 The evidence remains conflicting. Data indicate that roughly one-third of patients receiving LT will progress to the mCRPC stage, underscoring the importance of studying the impact of prior LT treatment on these patients. To date, limited evidence suggests that prior LT exposure in mCRPC patients significantly modifies the effect of subsequent intensified hormonal therapy using androgen deprivation therapy (ADT) plus ARPI.
Dr. Azem presented a meta-analysis of the COU-AA-3023 and ACIS4 trials using the YODA project database. Briefly, the COU-AA-302 trial randomized asymptomatic or mildly symptomatic patients with progressive, chemo-naïve mCRPC to receive either abiraterone acetate + prednisone or placebo + prednisone. Similarly, the ACIS trial randomized patients with mCRPC progressing on ADT without prior exposure to chemotherapy or ARPIs for CRPC to receive either apalutamide + abiraterone acetate + prednisone or placebo + abiraterone acetate + prednisone. The study design for both trials is illustrated below: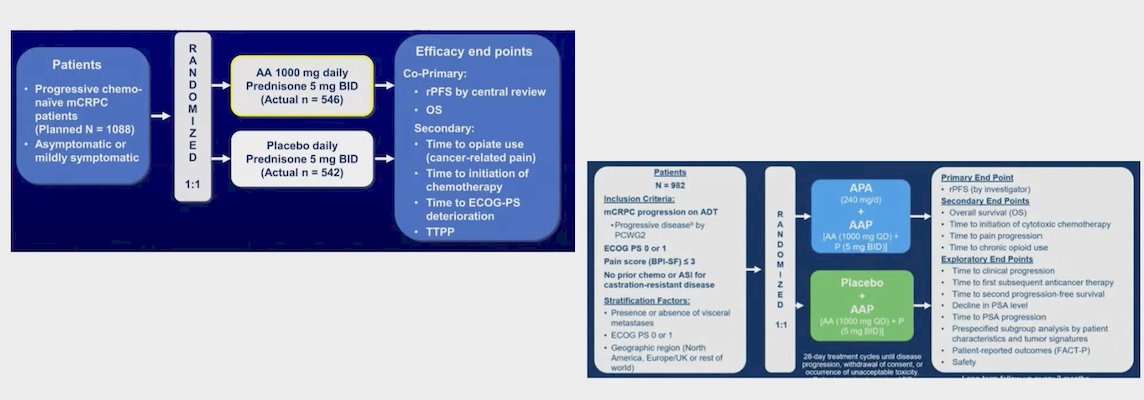
For the statistical analysis, the investigators applied a hierarchical multivariable Bayesian Cox proportional hazards regression model (CPHM) to determine if there was any difference in treatment effect on overall survival (OS) among patients stratified by prior local therapy (LT) in the COU-AA-302 and ACIS trials. They adjusted the association for fixed covariates, and study-level random variance, and tested the interaction between local therapy and OS. Hazard ratios (HR) with 95% confidence intervals (CIs) for the interaction term were reported. Additionally, a similar model without an interaction term was used to determine if there was an independent association of prior LT exposure with OS.
The investigators identified a total of 1,132 patients between both trials who received prior local therapy (LT) and 905 patients who did not receive any form of prior LT. Among patients with prior LT:
- 253 (22%) underwent radical prostatectomy (RP) alone
- 345 (30%) had RP plus radiation therapy (RT)
- 534 (48%) had RT
Among patients receiving some form of hormone intensification therapy, several had prior exposure to local therapy (LT). Specifically:
- Of the 1,025 patients who received abiraterone, 559 (55%) had received prior LT.
- Of the 485 patients who received abiraterone plus apalutamide, 237 (49%) had received prior LT.
- Among the 527 patients who received ADT alone, 338 (64%) had received prior LT.
In patients who received ADT + abiraterone + prednisone, the analysis showed no significant difference in overall survival (OS) between those with and without prior local therapy (LT). The hazard ratio (HR) for the interaction term was 0.92 (95% CI: 0.71-1.19), indicating no significant interaction between prior LT and treatment effect on OS.
Similarly, for patients who received apalutamide + abiraterone + prednisone compared to those who received ADT + abiraterone + prednisone, there was no significant difference in the treatment effect on OS among patients with and without prior LT. The HR for the interaction term was 1.25 (95% CI: 0.97-1.65).
The table below summarizes the differences among patients with and without prior LT when comparing these treatment arms: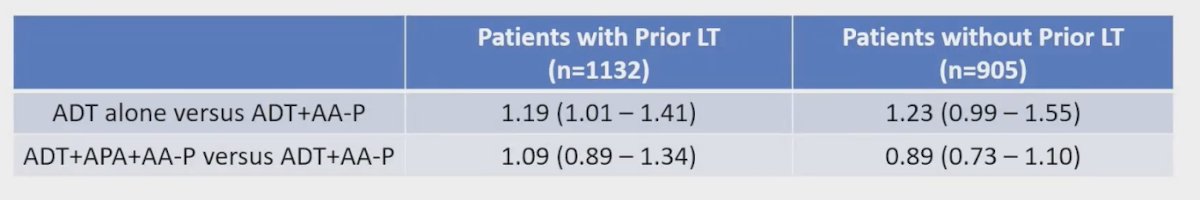
Exploring the association of prior LT modality with radiographic progression-free survival (rPFS) and OS, the investigators found no significant interaction between prior LT modality and either rPFS or OS. The table below illustrates these findings: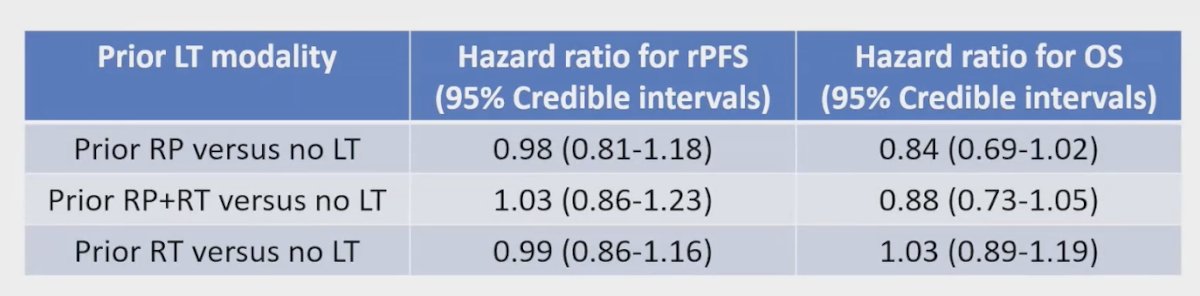
The study has several limitations that warrant consideration. Firstly, it is a post-hoc unplanned analysis, meaning that the findings are primarily hypothesis-generating rather than definitive conclusions. Additionally, treatment with local therapy (LT) was a non-randomized decision, introducing potential bias and unmeasured confounding factors not controlled for in the analysis. Consequently, the association between LT and outcomes like rPFS and OS may be influenced by these confounders. Moreover, the interpretation of the data is constrained by missing information on critical covariates such as PSA levels, Gleason scores, and T-stage at diagnosis. Lastly, this meta-analysis does not encompass all studies examining the role of first-line androgen receptor pathway inhibitors (ARPI) in the chemotherapy-naïve metastatic castration-resistant prostate cancer (mCRPC) setting, such as the PREVAIL trial.
To conclude his presentation, Dr. Azem highlighted the following key takeaways:
- In this meta-analysis, the investigators found no significant compromise in treatment effects from first-line ARPI in patients who received prior LT.
- It is reasonable to hypothesize that neuroendocrine differentiation induced by prior LT does not result in any clinically meaningful differences in treatment response to first-line ARPI in the mCRPC setting, at least based on the findings from the meta-analysis of these two trials.
- Exposure to prior LT showed no association with the risk of radiographic progression or overall mortality in this meta-analysis.
Presented by: Omar Azem, MD, PGY-3, Radiation Oncologist, Department of Radiation Oncology at Rush University Medical Center Chicago, IL
Written by: Julian Chavarriaga, MD – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @chavarriagaj on Twitter during the 2024 American Society for Radiation Oncology (ASTRO) annual meeting held in Washington D.C., between the 29th of September and the 2nd of October.
References- Deng X, Elzey BD, Poulson JM, Morrison WB, Ko SC, Hahn NM, Ratliff TL, Hu CD. Ionizing radiation induces neuroendocrine differentiation of prostate cancer cells in vitro, in vivo, and in prostate cancer patients. Am J Cancer Res. 2011;1(7):834-44. Epub 2011 Aug 18. PMID: 22016831; PMCID: PMC3196282.
- Roy S, Sun Y, Morgan SC, Wallis CJD, King K, Zhou YM, D'souza LA, Azem O, Cueto-Marquez AE, Camden NB, Spratt DE, Kishan AU, Saad F, Malone S. Effect of Prior Local Therapy on Response to First-line Androgen Receptor Axis Targeted Therapy in Metastatic Castrate-resistant Prostate Cancer: A Secondary Analysis of the COU-AA-302 Trial. Eur Urol. 2023 Jun;83(6):571-579. doi: 10.1016/j.eururo.2023.02.017. Epub 2023 Mar 7. PMID: 36894488.
- Ryan CJ, Smith MR, Fizazi K, Saad F, Mulders PF, Sternberg CN, Miller K, Logothetis CJ, Shore ND, Small EJ, Carles J, Flaig TW, Taplin ME, Higano CS, de Souza P, de Bono JS, Griffin TW, De Porre P, Yu MK, Park YC, Li J, Kheoh T, Naini V, Molina A, Rathkopf DE; COU-AA-302 Investigators. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015 Feb;16(2):152-60. doi: 10.1016/S1470-2045(14)71205-7. Epub 2015 Jan 16. PMID: 25601341.
- Saad F, Efstathiou E, Attard G, Flaig TW, Franke F, Goodman OB Jr, Oudard S, Steuber T, Suzuki H, Wu D, Yeruva K, De Porre P, Brookman-May S, Li S, Li J, Thomas S, Bevans KB, Mundle SD, McCarthy SA, Rathkopf DE; ACIS Investigators. Apalutamide plus abiraterone acetate and prednisone versus placebo plus abiraterone and prednisone in metastatic, castration-resistant prostate cancer (ACIS): a randomised, placebo-controlled, double-blind, multinational, phase 3 study. Lancet Oncol. 2021 Nov;22(11):1541-1559. doi: 10.1016/S1470-2045(21)00402-2. Epub 2021 Sep 30. PMID: 34600602; PMCID: PMC9377412.


