(UroToday.com) The 2024 American Society for Radiation Oncology (ASTRO) annual meeting held in Washington D.C. was host to the session GU Quick Pitch (GU 4). Dr. Qi Xin presented the 5-Year Results of the Prolong Study, evaluating Radiotherapy of the Primary Tumor and All Metastatic Lesions in Oligometastatic Prostate Cancer.
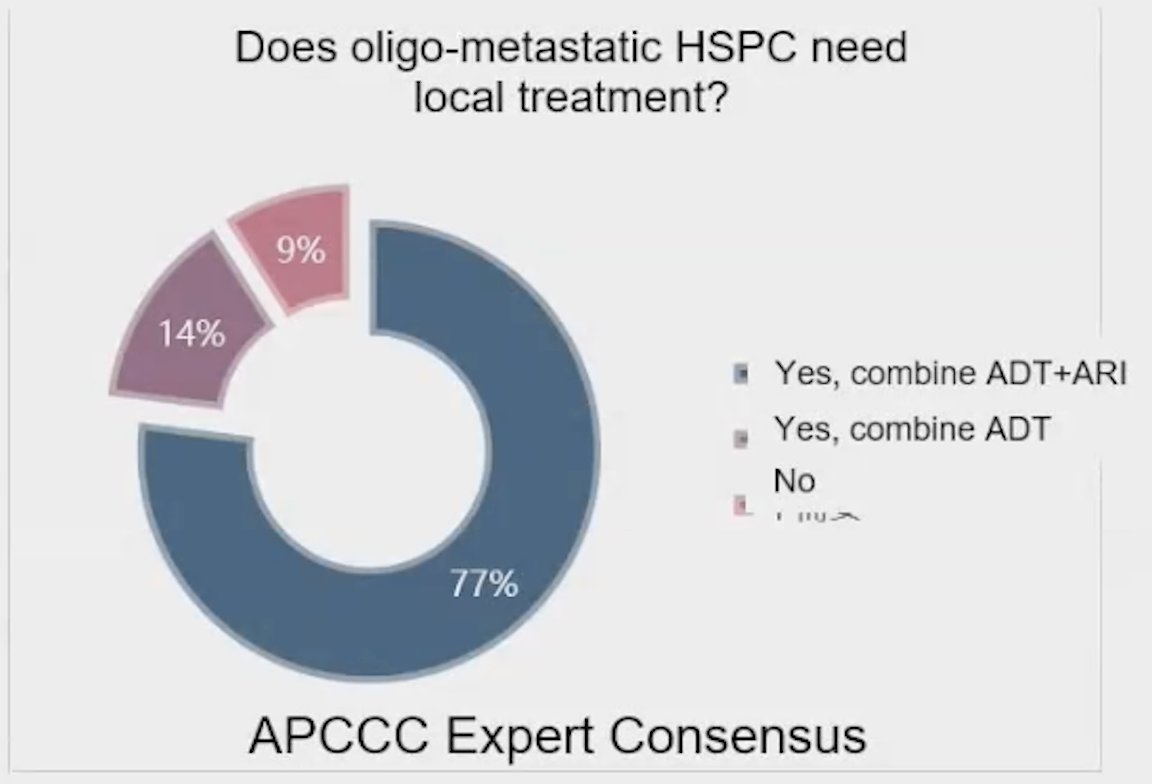
Dr. Xin began her presentation by highlighting the Advanced Prostate Cancer Consensus Conference (APCC) regarding the need for local treatment in oligometastatic hormone-sensitive prostate cancer (HSPC). She noted that 77% of the audience believed that local treatment, in combination with androgen deprivation therapy (ADT) and androgen receptor pathway inhibitors (ARPI), was necessary, while only 9% felt that these patients did not require any form of local treatment. This overwhelming support for local therapy suggests a shift in clinical thinking towards more aggressive management strategies in this patient population.
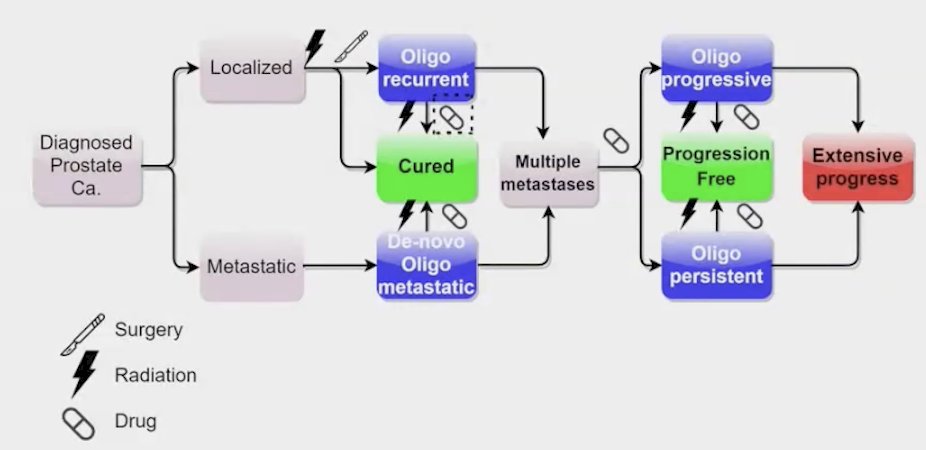
The Prolong study evaluated survival outcomes in patients with oligometastatic prostate cancer who were treated with radiotherapy (RT) targeting both the primary tumor and all metastatic lesions. The study included patients classified into several categories of oligometastatic disease:
- De novo oligometastatic: Patients who present with metastasis at diagnosis but have a limited number of metastatic sites.
- Oligo-recurrent: Patients who experience recurrence after initial treatment but present with a limited number of new metastatic lesions.
- Oligo-progressive: Patients who show progression of existing metastatic disease, again with a limited number of sites.
- Oligo-persistent: Patients with persistent metastatic lesions after initial treatment, classified as having a limited number of affected areas.
The Prolong study was a single-center cohort study conducted from October 2011 to January 2022, during which investigators accrued 395 patients with oligometastatic prostate cancer. These patients received radiotherapy (RT) targeting both the primary tumor and all metastatic lesions, in combination with androgen deprivation therapy (ADT) and androgen receptor pathway inhibitors (ARPI). Participants had the option to discontinue ADT if they achieved an undetectable prostate-specific antigen (PSA) level (less than 0.2 ng/ml) after two years of treatment.
Inclusion criteria for the cohort study included:
- A pathological diagnosis of prostate cancer.
- Imaging examinations revealing distant metastases, which could include non-regional lymph node metastases, bone metastases, or visceral metastases.
- The total number of metastases was limited to 10 or fewer, or the number of active metastases was limited to 10 or fewer after treatment.
The primary endpoint of the study was overall survival (OS), while key secondary endpoints included progression-free survival (PFS) (defined by PSA failure, local, or distant failure after RT), disease-specific survival (DSS), time to new metastases (TTNI), and local recurrence.
The most common fractionation scheme used in the Prolong study involved administering 70 Gy to the prostate in 25 fractions, while the metastatic sites received 37.5 Gy in 5 fractions. Following radiotherapy (RT), patients were monitored with prostate-specific antigen (PSA) tests every three months. Imaging evaluations were generally conducted every 6 to 12 months, although imaging should be performed immediately if clinical symptoms arose or if PSA levels continued to rise.
The median follow-up of the study was 60 months. A total of 264 patients (66.8%) had HSPC, while 131 patients (33.2%) had CRPC. Among the patients, 56% had de novo oligo-metastatic disease, followed by 27% with oligo-progressive disease, 15% with oligo-recurrent disease, and 2% with oligo-persistent disease. The median age of the cohort was 69 years.
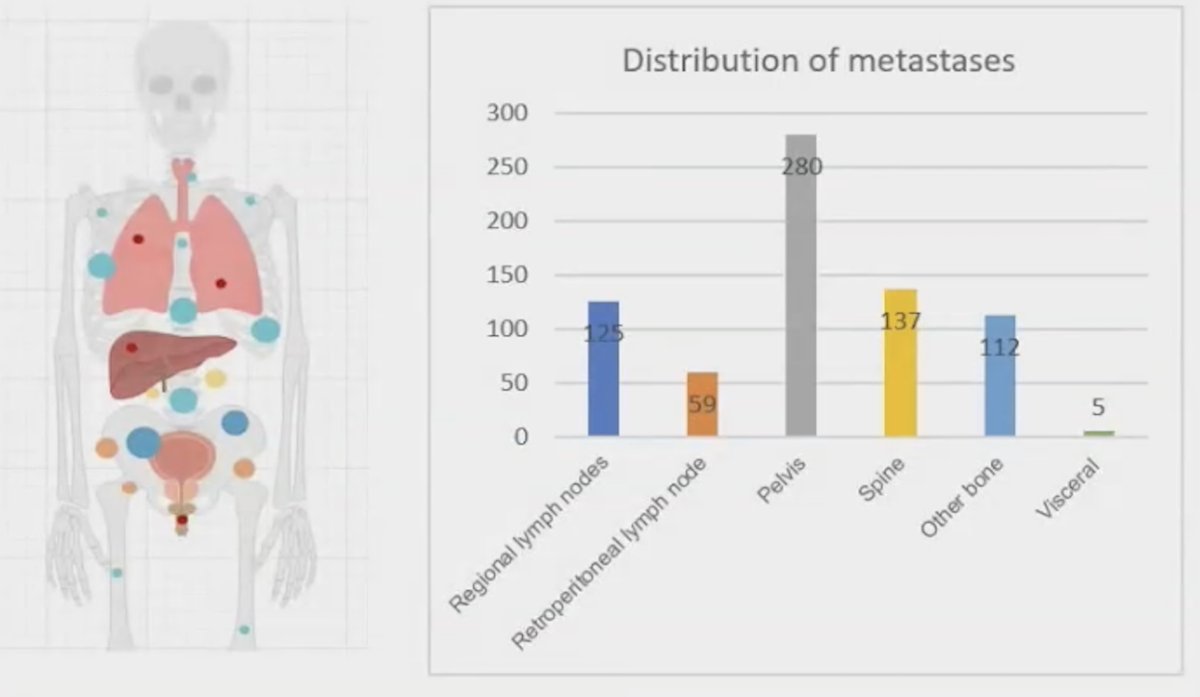
A total of 718 metastases received radiotherapy, with 529 (73.7%) being bone metastases, 125 (17.4%) involving regional lymph nodes, and 59 (8.2%) in the retroperitoneal lymph nodes, as illustrated in the graphic below.
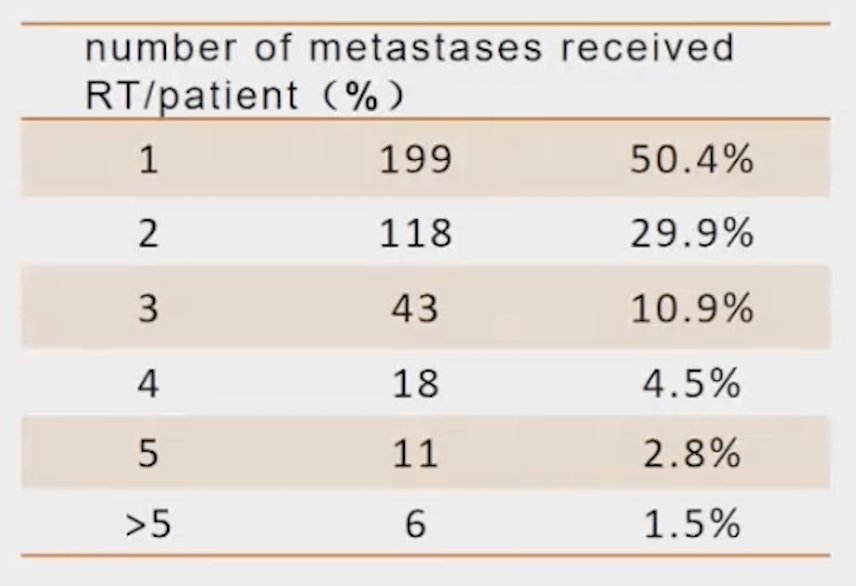
The number of metastases that received RT per patient is shown in the table below. Most patients had either one (50.4%) or two (29.9%) metastatic sites receiving RT. The median biologically effective dose (BED3) was 121.3Gy (IQR: 113.3-131.3Gy).
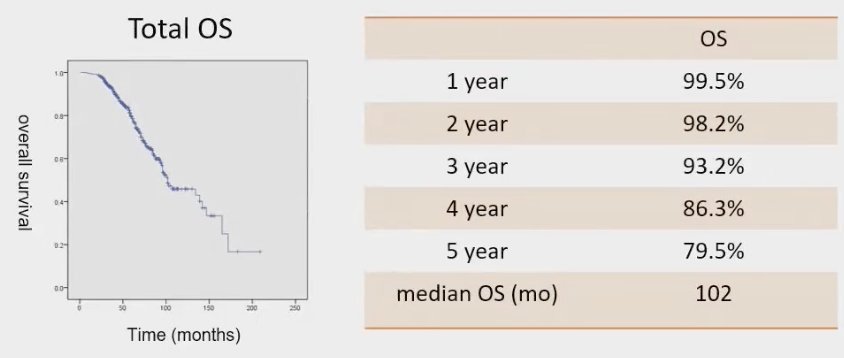
For the whole cohort, the OS was 99.5% at 1 year, 93.2% at 3 years and 79.5% at 5 years. The median OS was 102 months.
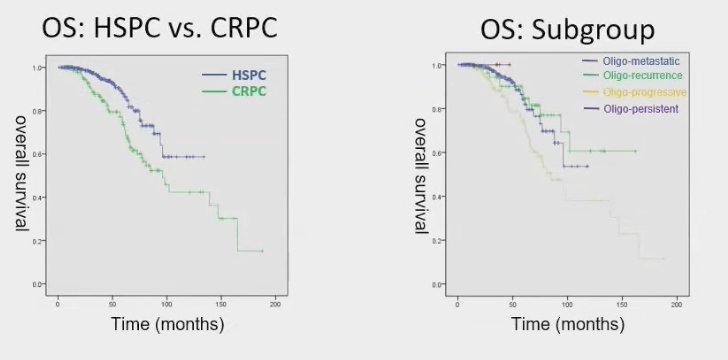
The median progression-free survival (PFS) in patients with HSPC was not reached, whereas men with CRPC had a median PFS of 10 months. Univariate analysis indicated that factors associated with improved PFS in HSPC included HSPC itself, lower metastatic volume, a shorter interval between diagnosis and RT, higher PSA at diagnosis, the use of whole pelvic radiotherapy (WPRT), lower pre-RT PSA, the type of ADT used, lower Gleason scores, and the use of chemotherapy.
Notably, subgroup analysis for OS revealed that patients with CRPC and those with oligo-progressive disease experienced inferior OS outcomes. This suggests that these patient populations may require closer monitoring and potentially different therapeutic approaches to improve survival rates.
Dr Xin, concluded her presentation with the following take home messages:
- Total coverage radiotherapy for oligo-metastatic prostate cancer is safe for multiple groups of oligo-metastatic prostate cancer.
- Compared with the published survival data for metastatic prostate cancer, overall survival has achieved good results. In the PROLONG study
- For patients with oligo-metastatic prostate cancer, RT should be done as soon as possible, and total covered radiotherapy is recommended in the stage of HSPC patients.
- This trial may help to guide further randomized controlled trial design evaluating the duration of drugs (ADT+ARPI) for oligo-metastatic prostate cancer (i.e. using undetectable PSA<0.2 ng/ml at 2 years as a criterion for stopping ADT+ARPI)
Presented by: Qi Xin, MD, MS, Peking University First Hospital, Beijing, China.
Written by: Julian Chavarriaga, MD – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @chavarriagaj on Twitter during the 2024 American Society for Radiation Oncology (ASTRO) annual meeting held in Washington D.C., between the 29th of September and the 2nd of October.


