(UroToday.comThe 2024 American Society for Radiation Oncology (ASTRO) annual meeting held in Washington D.C., between September 29 and October 2 was host to the session GU Quick Pitch (GU 4). Dr. Devaki Shilpa Surasi presented A Prospective Pilot Study Investigating 18F rhPSMA-7.3 PET/MRI to Detect Recurrent Disease and Guide Radiotherapy Planning in Patients with Biochemically Recurrent Prostate Cancer Post-Prostatectomy.
Dr. Surasi began her presentation by discussing the challenges associated with detecting small-volume metastatic disease using conventional imaging methods in the context of biochemical recurrence of prostate cancer (PCa). Simultaneous positron emission tomography/magnetic resonance imaging (PET/MRI) offers several advantages by combining metabolic information from the PET with the high spatial resolution of the MRI. This combination should allow for more accurate identification of disease compared to traditional conventional imaging.
F-18 rhPSMA 7.3 (rhomboid human prostate-specific membrane antigen 7.3) ligands represent a new class of diagnostic and therapeutic agents targeting prostate-specific membrane antigen in PCa. These agents received approval from the U.S. Food and Drug Administration (FDA) in 2023. The investigators hypothesized that F-18 rhPSMA 7.3 PET/MRI could accurately detect recurrent PCa and assist in designing treatment fields for salvage radiation therapy (RT) planning, even at low prostate-specific antigen (PSA) levels.
This was a prospective pilot study (NCT04978675) that enrolled men with biochemical recurrence after prostatectomy, defined as having a prostate-specific antigen (PSA) level of 0.2 ng/mL on two confirmatory measurements. The study involved rhPSMA 7.3 PET/MRI conducted between August 2021 and January 2023 on a simultaneous 3T PET/MRI scanner.
Patients with positive rhPSMA 7.3 PET/MRI scans returned for a follow-up PET/MRI scan after treatment, scheduled 6 to 18 months after the initial scan. Radiation oncologists documented any changes to the radiation therapy (RT) plan based on the results of the PET/MRI scans. All patients underwent standard fractionated RT along with a minimum of six months of androgen deprivation therapy (ADT).
The investigators established the standard of truth using pathology when feasible or through a combination of confirmatory imaging that showed radiographic and PSA responses after treatment.
The primary endpoint of the study was:
- To evaluate the positive predictive value (PPV) of rhPSMA 7.3 PET/MRI in detecting disease recurrence.
The secondary endpoint was:
- To assess changes in the RT plan following rhPSMA 7.3 PET/MRI and evaluate treatment responses after any modifications.
A total of 29 patients were enrolled in the study. Of these, 28 underwent rhPSMA 7.3 PET/MRI at the first time point, and 20 had a positive scan. Sixteen patients completed a second rhPSMA 7.3 PET/MRI scan, and 15 of these were determined to be true positives based on the established criteria.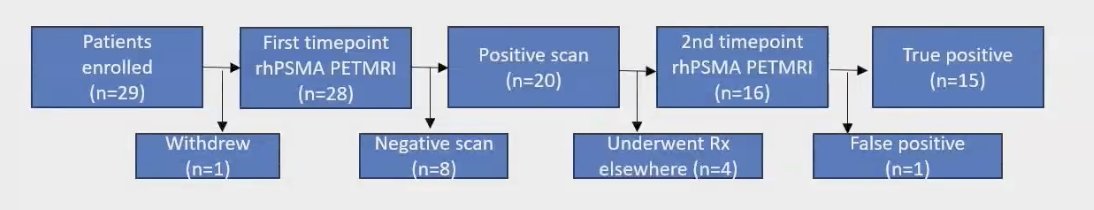
The median age of the cohort was 66 years, with a median prostate-specific antigen (PSA) level before surgery of 7.03 ng/mL. The median PSA before the first rhPSMA 7.3 PET/MRI scan was 0.3 ng/mL (interquartile range [IQR] 0.2-1.5). The time interval between surgery and PSA recurrence was 19.4 months. Full details are provided in the table below:
The overall positive predictive value (PPV) rate for rhPSMA 7.3 PET/MRI in detecting disease recurrence was 93.75%, with 15 out of 16 patients accurately identified as having a recurrence. Notably, the PPV for local recurrence in the prostate bed, as well as in pelvic and extrapelvic lymph nodes, reached an impressive 100%. The PPV rates by lesion site are detailed below: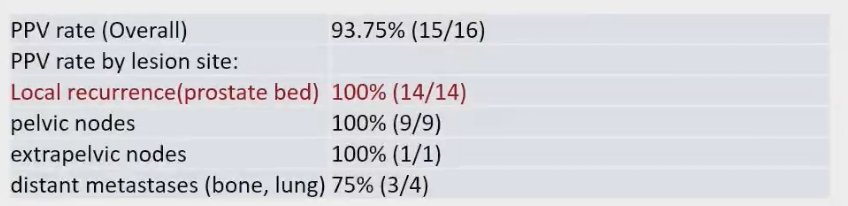
F-18 rhPSMA 7.3 PET/MRI demonstrated a high detection rate of true positive lesions, even at low PSA The PPV for patients with a PSA level of 0.2 ng/mL or less than 0.5 ng/mL was recorded at 92.3%. The PPV rates categorized by PSA level are detailed below: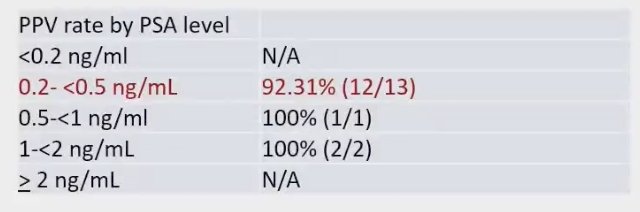
After the F-18 rhPSMA 7.3 PET/MRI scans, significant treatment changes were observed in 8 out of 22 patients (36%). These major changes included:
- Extension of Clinical Target Volumes (CTV): The CTVs were adjusted to encompass PSMA-positive lesions within the pelvis.
- Superior Extension of CTVs: The CTVs were extended to include paraaortic lymph nodes.
- Addition of Metastasis-Directed Stereotactic Body Radiation Therapy (SBRT): This was implemented for patients with extrapelvic oligometastatic disease, defined as 1-5 extrapelvic sites classified as M1a or M1b.
- Radiotherapy Deemed Futile: In cases with visibly polymetastatic disease (more than 5 M1a or M1b sites) or visceral metastatic disease (M1c), radiotherapy was considered unnecessary.
A total of 9 (41%) had minor changes that were PSMA-positive lesions covered within the CTV (potential for dose escalation to gross disease or dose de-escalation to the rest of the prostate fossa) Notably, all the patients who underwent combination of RT and ADT had a complete response on the second timepoint of the F-18 rhPSMA 7.3 PET/MRI (n=16).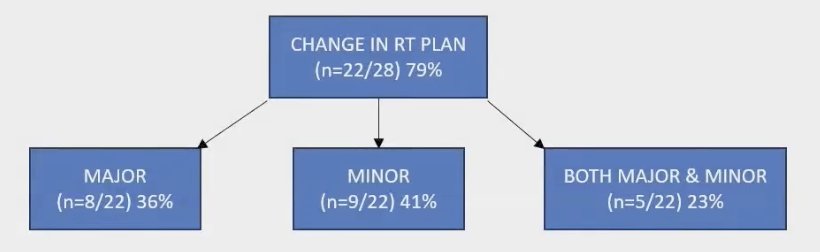
Dr. Surasi concluded her presentation by discussing a patient who had undergone radical prostatectomy and subsequently presented with a prostate-specific antigen (PSA) level of 0.3. While either the PET alone or the MRI alone detected fibrotic changes potentially attributed to post-surgical effects, the rhPSMA 7.3 PET/MRI provided clear evidence of recurrence in the prostatic fossa. This detection was facilitated by the enhanced spatial resolution offered by the combination of PET and MRI, as illustrated in the images presented below.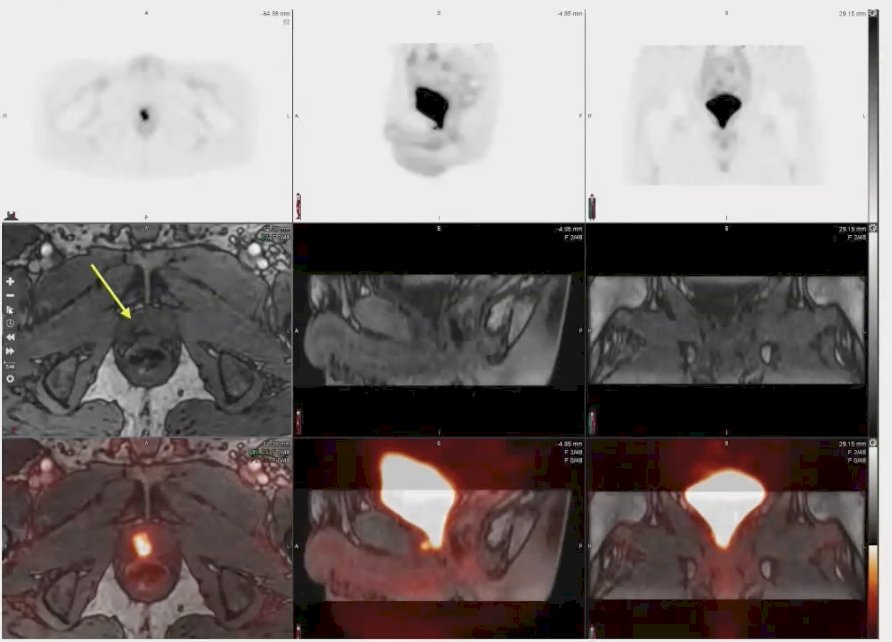
The conclusions of this presentation were:
- F-18 rhPSMA 7.3 PET/MRI resulted in a high detection rate of true positive lesions even at low PSA levels with changes of RT plans in 79% of the patients.
- Simultaneous F-18 rhPSMA 7.3 PET/MRI imaging can potentially be used as a "one-stop shop" to stratify patient treatment and tailor salvage radiation fields.
Presented by: Devaki Shilpa Surasi, MD, MBBS, Associate Professor, Department of Nuclear Medicine, Division of Diagnostic Imaging, The University of Texas MD Anderson Cancer Center, Houston, TX
Written by: Julian Chavarriaga, MD – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @chavarriagaj on Twitter during the 2024 American Society for Radiation Oncology (ASTRO) annual meeting held in Washington D.C., between the 29th of September and the 2nd of October.


