(UroToday.com) The 2024 American Society for Radiation Oncology (ASTRO) annual meeting held in Washington D.C., between September 29 and October 2 was host to the session GU Quick Pitch (GU 4). Dr. Savita Dandapani presented the Initial Results of a Phase 2 Trial of Stereotactic Body Radiation Therapy, Hormone/Androgen Deprivation Therapy, and Radium 223 Dichloride for Oligometastatic Castrate Sensitive Prostate Cancer (SHARP).
Dr. Dandapani began her presentation by discussing the paradigm for treating oligometastatic prostate cancer with stereotactic body radiation therapy (SBRT) alone. Oligometastatic disease is typically defined as having fewer than five metastases. Numerous studies have shown that SBRT can improve progression-free survival (PFS) and delay the initiation of androgen deprivation therapy (ADT), thereby enhancing health-related quality of life.
Key phase II studies, including SABR-COMET, POPSTAR, STOMP, and ORIOLE, illustrate the benefits of incorporating SBRT early in the management of metastatic hormone-sensitive prostate cancer (mHSPC). These studies suggest that early intervention with SBRT can lead to improved outcomes, demonstrating its role in changing the treatment landscape for patients with oligometastatic disease.
Despite numerous trials outlining the benefits of early stereotactic body radiation therapy (SBRT), there is a notable lack of data on the efficacy of radiopharmaceuticals, both alone and in combination with SBRT for treating tumors. Bone metastases account for up to 90% of all prostate cancer metastases, and while SBRT can effectively target gross tumors visible on imaging, a critical question arises: how can we enhance the outcomes of SBRT trials? Specifically, how can we address the clinical dilemma of treating micrometastases?
One promising avenue is the use of radiopharmaceuticals as a form of "systemic radiation." Radium-223 (Xofigo), an alpha-emitting radiopharmaceutical, is known for its shorter penetration range, which can minimize damage to the bone marrow. Recognizing the potential of this approach, Dr. Dandapani proposed an investigator-initiated trial concept to Bayer in 2015. The SHARP trial (NCT03361735) aims to investigate the synergy between SBRT and radiopharmaceuticals (Radium-223) in treating oligometastatic prostate cancer, potentially enhancing patient outcomes. The investigators hypothesized that an aggressive treatment strategy combining androgen deprivation therapy (ADT), SBRT, and Radium-223 (Ra-223) could improve treatment outcomes by delaying disease progression and the onset of castration-resistant disease.
This approach is supported by three mechanisms:
- SBRT: Ablates gross tumors, effectively reducing the tumor burden.
- Radium-223: Targets micrometastases, controlling disease spread at the microscopic level.
- ADT (for 9 months): Works to ablate both gross tumors and micrometastases.
The primary outcome of the SHARP trial was progression-free survival (PFS), specifically measuring the time to treatment failure (TTF). Key secondary endpoints included delaying the need for systemic treatment, assessing health-related quality of life (HRQoL), and identifying biological correlatives for response, such as cytokines and circulating tumor DNA (ctDNA).
In this trial design, patients received AD) for 36 weeks alongside 7 cycles of Radium-223. Stereotactic body radiation therapy (SBRT) was administered on cycle 1, day 1, with a tailored approach: 9 Gy delivered in 3 fractions to bone lesions, 5 Gy in 5 fractions to lymph node lesions, and 10 Gy in 5 fractions to lung lesions. The study design is shown below: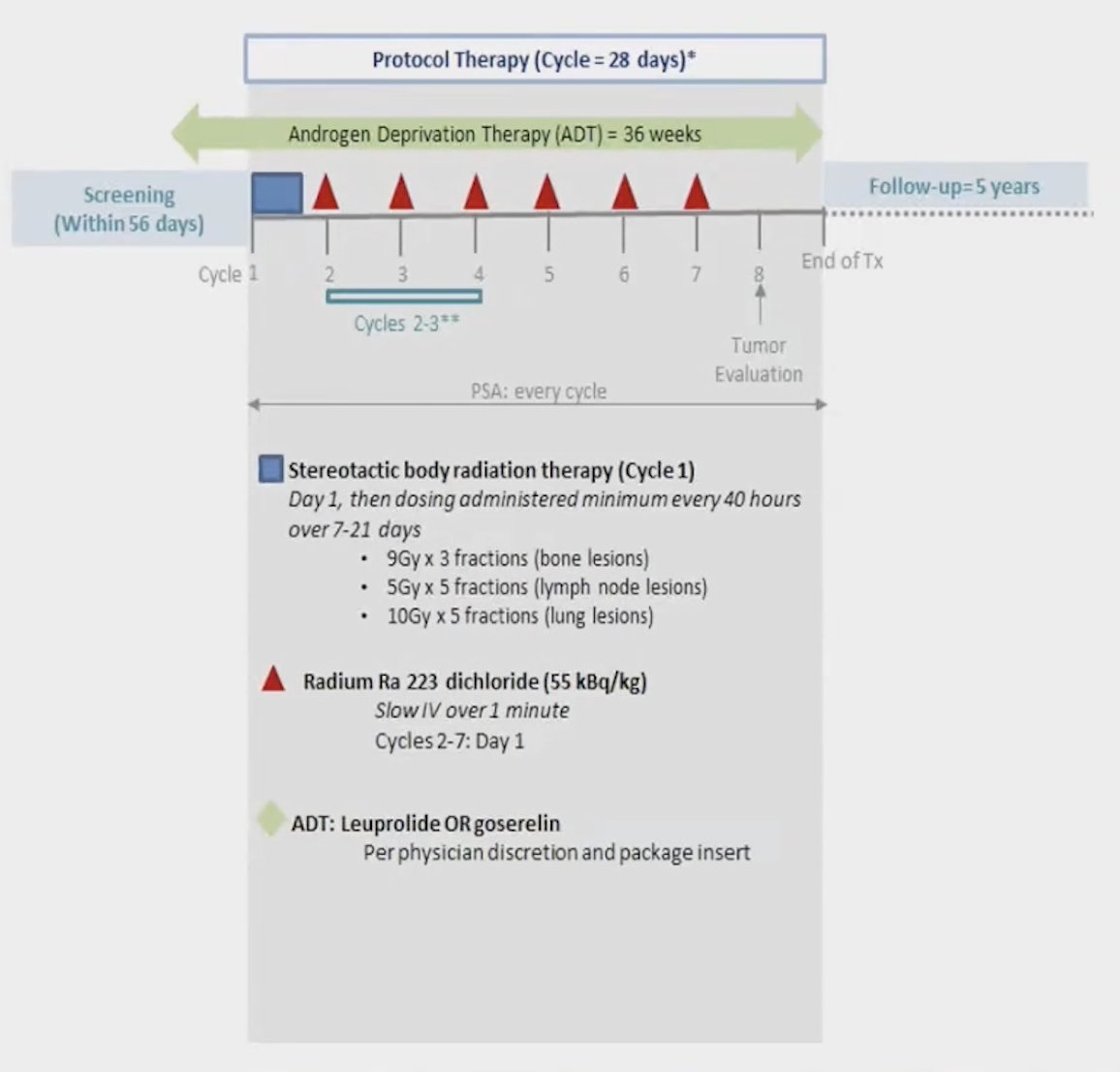
The median overall survival was not reached in this study, with only 3 events reported among the 28 evaluable patients. When comparing patients with de novo oligometastatic disease to those with oligoprogressive disease, the median OS for both groups was also not reached (Log-rank p=0.4). However, visual analysis of the survival curves suggests that patients with de novo oligometastatic disease appear to fare better than those with oligoprogressive disease.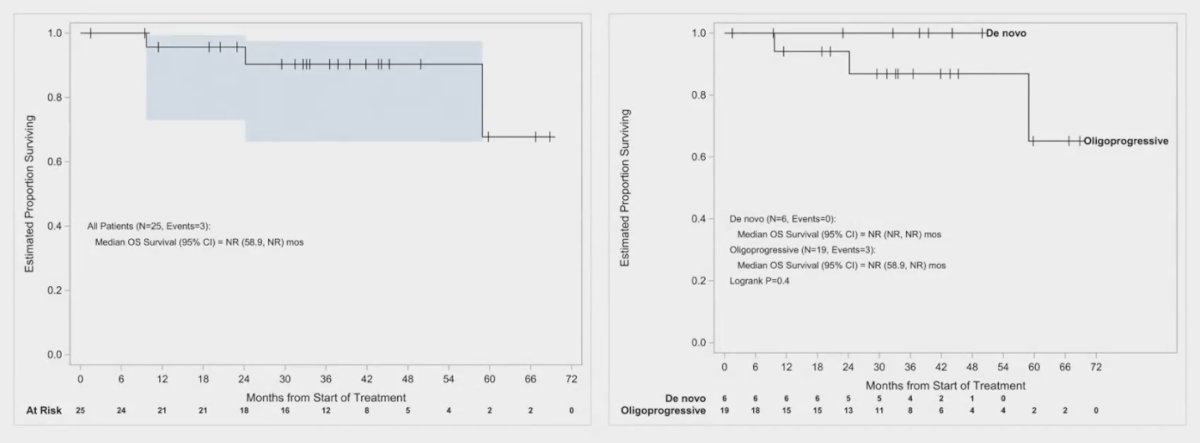
In the overall population, the median progression-free survival (PFS) was 21.7 months (range 13.8 to 49.9 months). For patients with de novo oligometastatic disease, the median PFS was significantly better at 34.3 months, while those with oligoprogressive disease had a median PFS of only 19 months.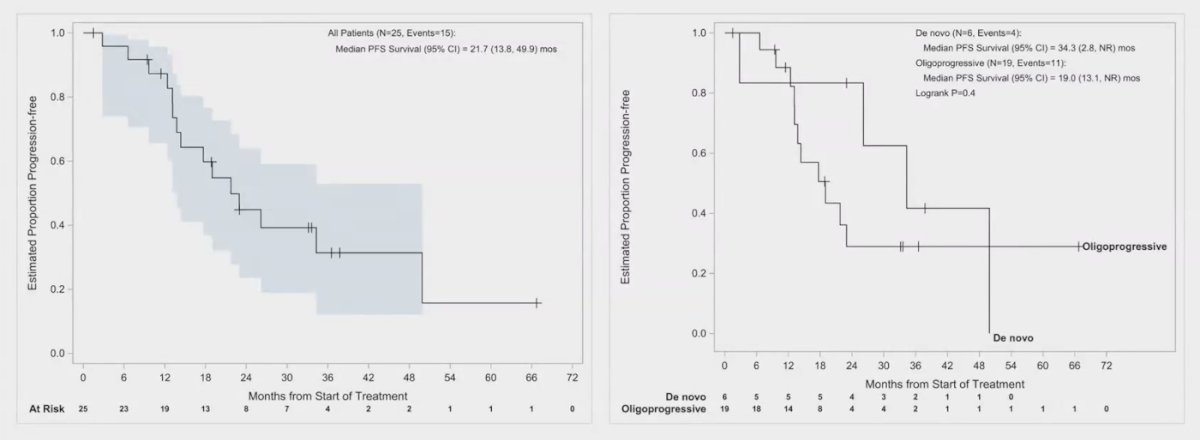
The initial biomarker analysis of the SHARP trial revealed notable changes in cytokine levels following treatment. Specifically, interleukin-1 beta (IL-1β) and tumor necrosis factor-alpha (TNF-α) cytokines decreased after the first cycle, indicating a potential anti-inflammatory response. Interestingly, interleukin-8 (IL-8) levels increased after this initial treatment cycle. This rise in IL-8 could suggest a compensatory mechanism or a different aspect of the inflammatory response that warrants further investigation.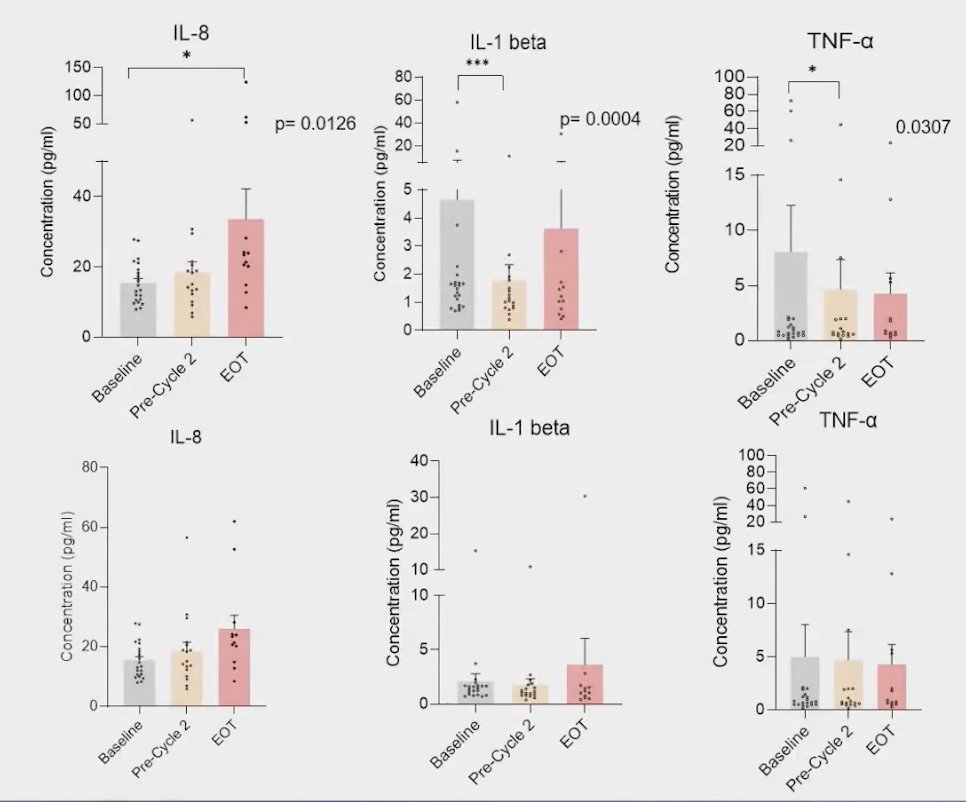
Dr. Dandapani concludes her presentations with the following key messages:
Radium-223 improved PFS and delayed TTF compared to historical controls receiving SBRT alone.
- Patients with de novo oligometastatic disease had better PFS and OS compared to those with oligoprogressive disease.
- Future directions include comparing Radium-223 (Xofigo) and Lu177-PSMA (Pluvicto) in the AlphaBet study, focusing on mHSPC, and completing biomarker analysis to predict patient benefit from this approach.
- A secondary analysis of time-to-bone metastatic development with the early addition of Radium-223 to SBRT is ongoing.
Presented by: Savita Dandapani, MD, PhD, Radiation Oncologist, City of Hope Comprehensive Cancer Center, Duarte, CA
Written by: Julian Chavarriaga, MD – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @chavarriagaj on Twitter during the 2024 American Society for Radiation Oncology (ASTRO) annual meeting held in Washington D.C., between the 29th of September and the 2nd of October.
References:- Palma DA, Olson R, Harrow S, Gaede S, Louie AV, Haasbeek C, Mulroy L, Lock M, Rodrigues GB, Yaremko BP, Schellenberg D, Ahmad B, Senthi S, Swaminath A, Kopek N, Liu M, Moore K, Currie S, Schlijper R, Bauman GS, Laba J, Qu XM, Warner A, Senan S. Stereotactic Ablative Radiotherapy for the Comprehensive Treatment of Oligometastatic Cancers: Long-Term Results of the SABR-COMET Phase II Randomized Trial. J Clin Oncol. 2020 Sep 1;38(25):2830-2838. doi: 10.1200/JCO.20.00818. Epub 2020 Jun 2. PMID: 32484754; PMCID: PMC7460150.
- Deek MP, Van der Eecken K, Sutera P, Deek RA, Fonteyne V, Mendes AA, Decaestecker K, Kiess AP, Lumen N, Phillips R, De Bruycker A, Mishra M, Rana Z, Molitoris J, Lambert B, Delrue L, Wang H, Lowe K, Verbeke S, Van Dorpe J, Bultijnck R, Villeirs G, De Man K, Ameye F, Song DY, DeWeese T, Paller CJ, Feng FY, Wyatt A, Pienta KJ, Diehn M, Bentzen SM, Joniau S, Vanhaverbeke F, De Meerleer G, Antonarakis ES, Lotan TL, Berlin A, Siva S, Ost P, Tran PT. Long-Term Outcomes and Genetic Predictors of Response to Metastasis-Directed Therapy Versus Observation in Oligometastatic Prostate Cancer: Analysis of STOMP and ORIOLE Trials. J Clin Oncol. 2022 Oct 10;40(29):3377-3382. doi: 10.1200/JCO.22.00644. Epub 2022 Aug 24. PMID: 36001857; PMCID: PMC10166371.
- Siva S, Bressel M, Murphy DG, Shaw M, Chander S, Violet J, Tai KH, Udovicich C, Lim A, Selbie L, Hofman MS, Kron T, Moon D, Goad J, Lawrentschuk N, Foroudi F. Stereotactic Abative Body Radiotherapy (SABR) for Oligometastatic Prostate Cancer: A Prospective Clinical Trial. Eur Urol. 2018 Oct;74(4):455-462. doi: 10.1016/j.eururo.2018.06.004. Epub 2018 Jun 29. PMID: 30227924.


