(UroToday.com) The 2024 American Society for Radiation Oncology (ASTRO) Annual Meeting held in Washington, DC between September 29th and October 2nd, 2024, was host to a presidential symposium of innovations in genitourinary cancers, specifically addressing radiotherapy innovations for prostate cancer that can be implemented in contemporary practice. Dr. Phuoc Tran discussed the ‘space-time continuum’ in metastatic castrations-sensitive prostate cancer (mCSPC), noting the following key points:
- Space and Time are actually clinical manifestations (i.e., phenotypes) of underlying metastatic biology.
- Understanding underlying metastatic biology better can instruct the rationale design of combination systemic and local therapy for metastatic disease.
- Local therapists can benefit metastatic prostate cancer patients now (beyond palliation)
What is the ‘space-time continuum’ in mCSPC? Historically, patients were considered curable only if they had localized disease, and the ‘cure line’ separated those with curable disease from those with incurable metastatic disease, including those with low metastatic burdens.
However, recently, the concept of oligometastatic disease has been adopted with the emergence of the spectrum or continuum metastatic theory, with the ‘cure line’ now shifted to potentially include patients with oligometastatic (i.e., limited) disease.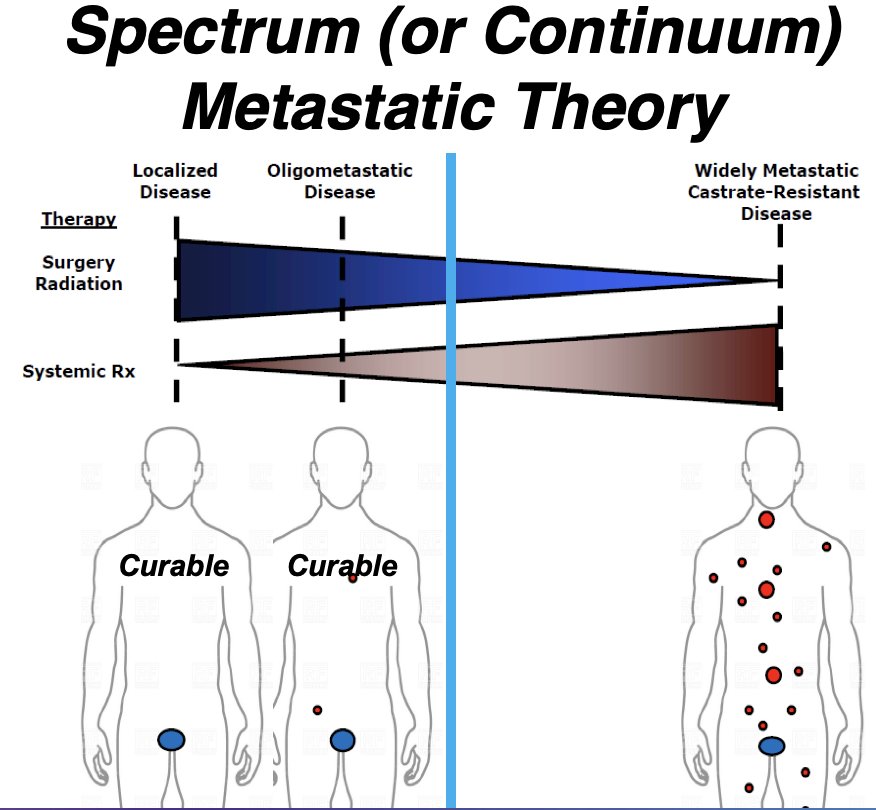
The oligometastatic hypothesis was coined by S. Hellman and R. R. Weichselbaum. It refers to an intermediate state of cancer spread between localized disease and widespread metastases that can be impacted by local therapies. He noted that metastasis is considered a clonal process whereby patients can undergo oligoclonal progression from an oligometastatic to a polymetastatic state.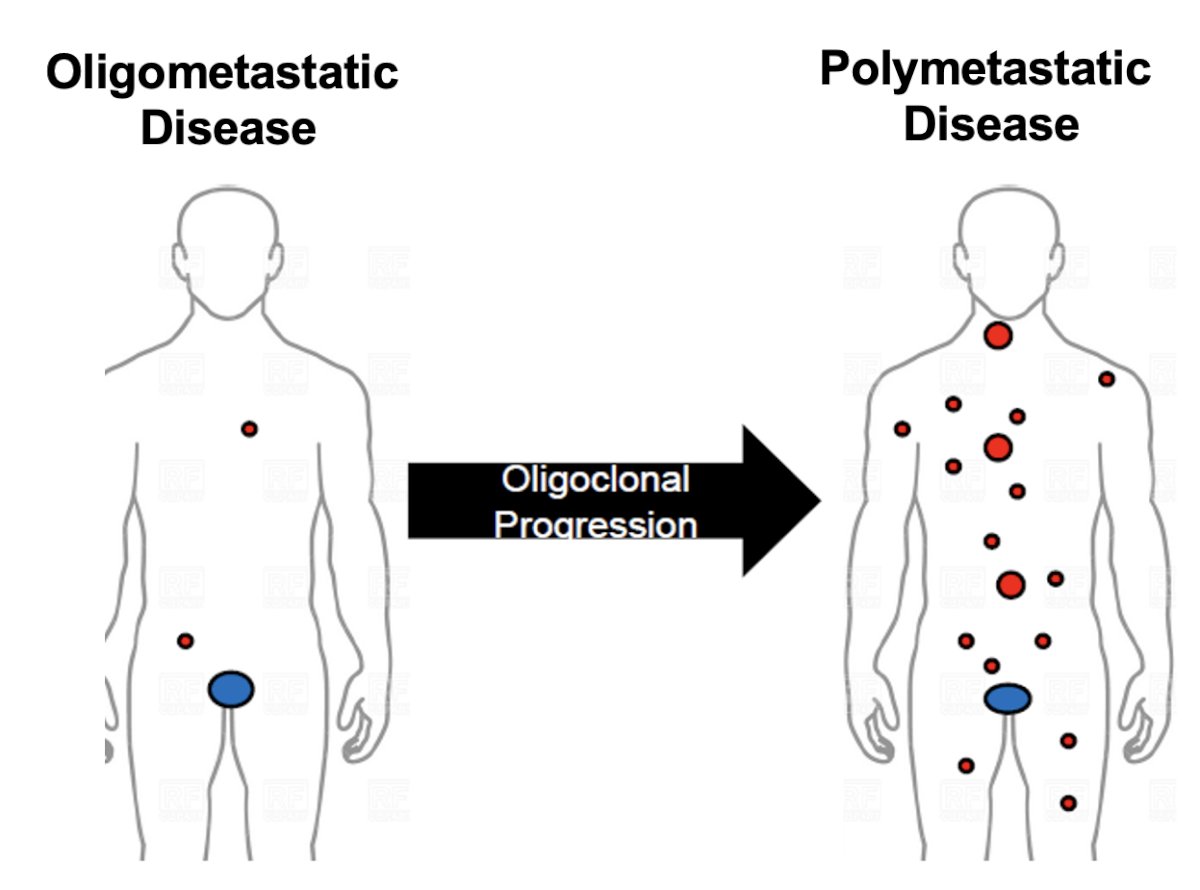
The self-seeding and clonal progression hypothesis theorizes that:
- Metastatic cells can cycle back to the primary tumor or self-seed
- Both the primary tumor and existing metastases can seed new metastases
- To change the natural history of oligometastatic disease, one may need to ablate both the primary and metastatic lesions

Another suggested concept is the sub-consolidative hypothesis, which states that if oligoprogression is a pathway to widely metastatic disease, then consolidative local therapies can alter this natural history.
This is reinforced by the results of the STAMPEDE Arm H trial that demonstrated that volume of metastatic disease (i.e., space) matters. Patients with CHAARTED low-volume disease derived a significant overall survival benefit from the addition of prostate radiotherapy to systemic therapy (3-year overall survival: 65% versus 53%, p<0.01), whereas patients with CHAARTED high-volume disease did not derive a significant survival benefit (p=0.16).1
Additional data from the STAMPEDE Arm H trial demonstrated that the benefit of prostate radiotherapy diminishes as the number of metastatic lesions increase, with prostate radiotherapy best reserved for patients with ≤5 lesions.2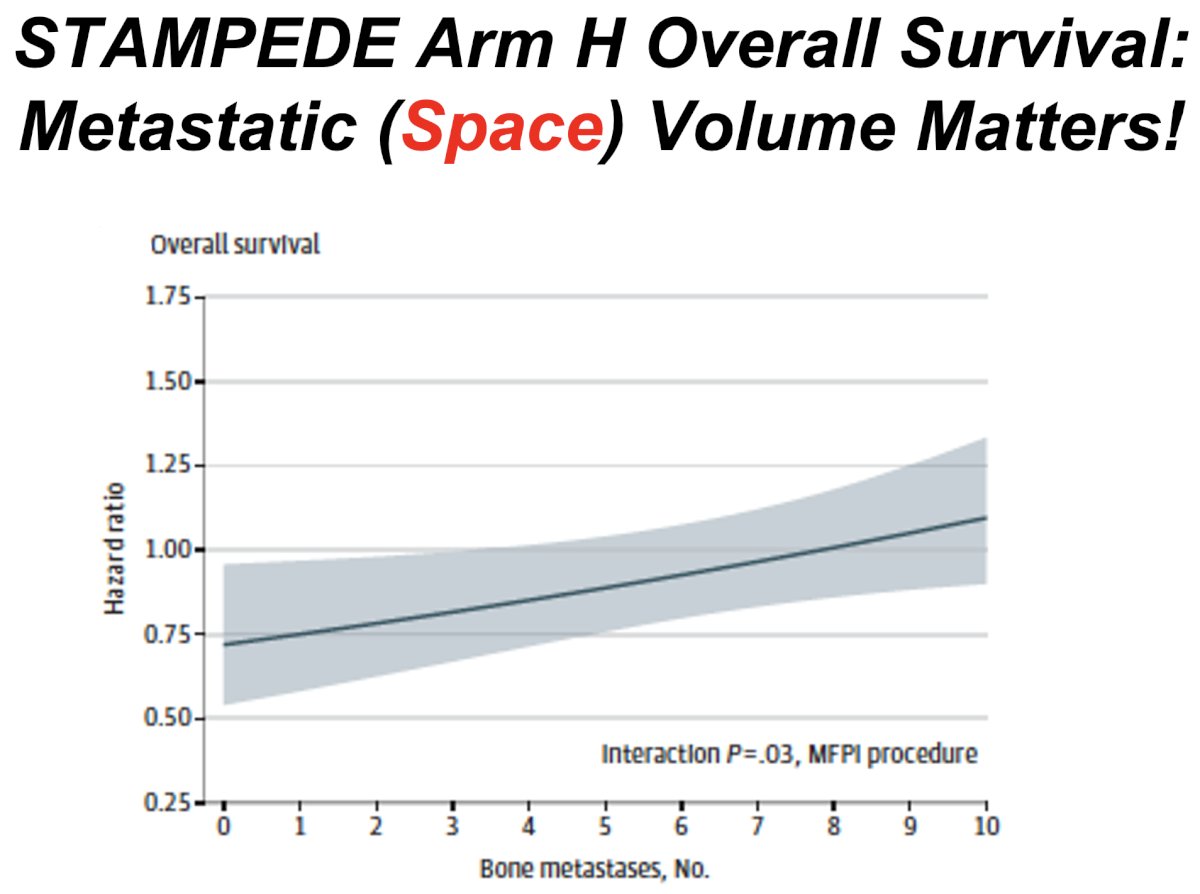
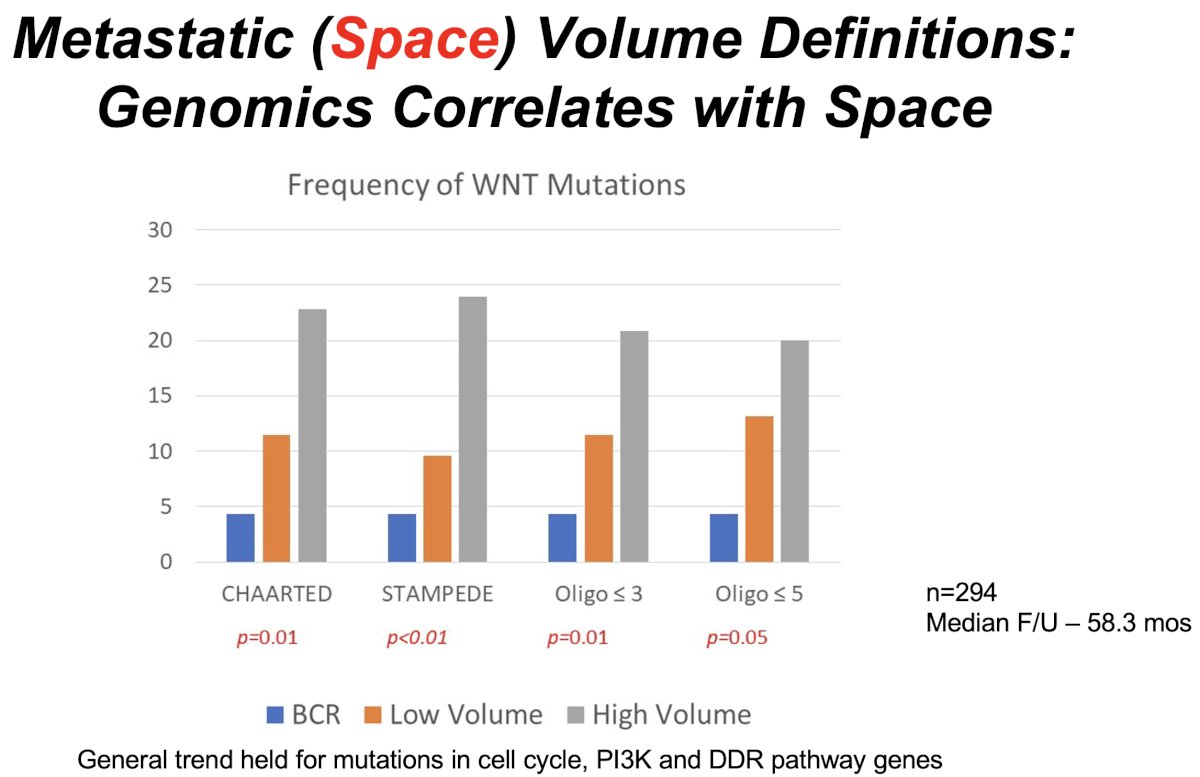
Importantly, there appear to be genomic correlates with the volume of metastatic disease. In 2023, Sutera et al. published the results of a retrospective review of patients with biochemically recurrent or mCSPC whose tumors underwent somatic targeted sequencing. 294 patients were included and followed for a median of 58.3 months. The frequency of driver mutations in WNT, cell cycle, TP53, and PI3K/AKT/mTOR were significantly different between biochemically recurrent, low-, and high-volume disease, with a higher incidence of mutations in patients with higher volumes of metastatic disease.3
The total consolidative hypothesis incorporates the use of MDT combined with systemic therapy in patients with oligometastatic disease. TERPs (NCT05223803) is a phase II trial of patients with de novo oligometastatic disease (<3 on conventional imaging or <5 on PET/CT) who will be randomized 1:1 to best systemic therapy + primary prostate radiotherapy +/- MDT (SBRT). The primary study endpoint is two-year failure-free survival.
In summary, for patients with de novo oligometastatic CSPC, Dr. Tran noted that there is level 1 data from STAMPEDE and PEACE-1 to support the use of local treatment intensification with external beam radiotherapy for the primary tumor. However, the evidence for combining MDT (SBRT) to metastatic lesions with systemic therapy remains weak, with phase II single-arm studies ongoing in this space.
Next, addressing the ‘time’ component of the ‘space-time continuum’, Dr. Tran noted that oligometastatic patients may present with either de novo (i.e., synchronous) or recurrent (i.e., metachronous) disease. This is of particular clinical significance given that patients with synchronous metastases have significantly worse overall survival outcomes (5-year overall survival: 39% versus 79%, p<0.01).4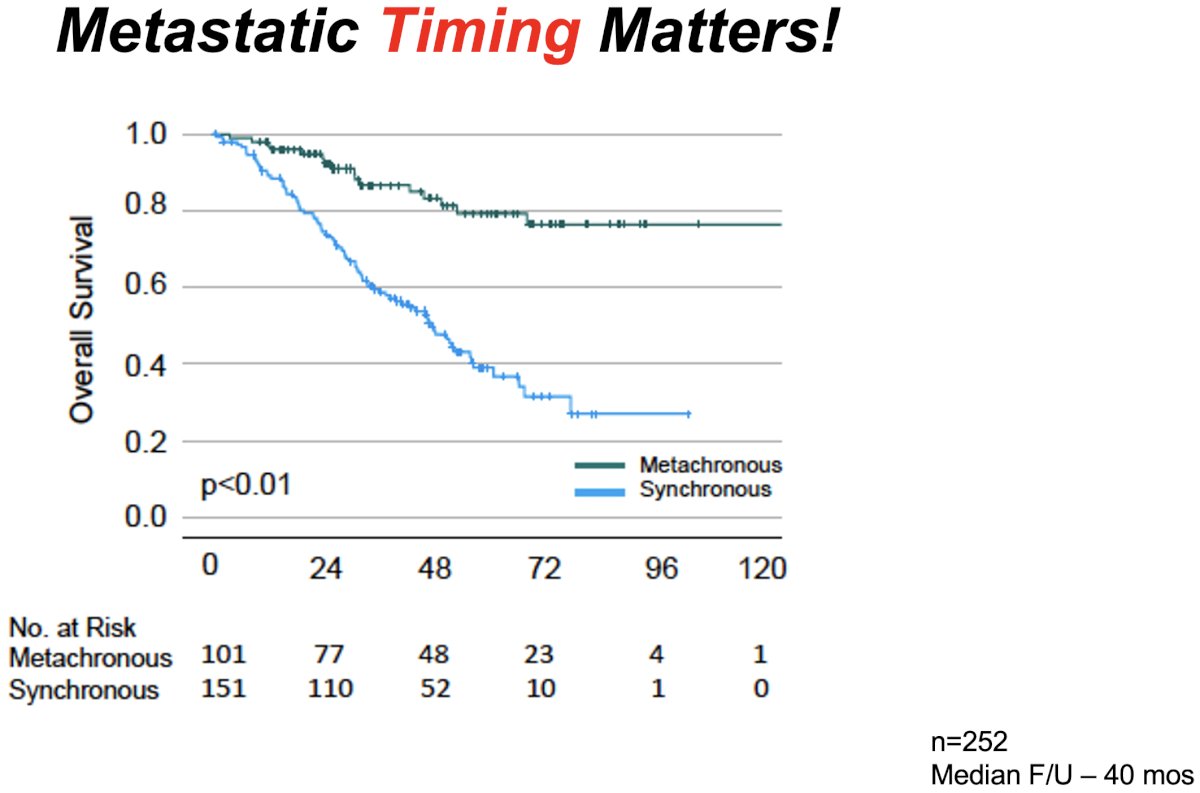
The timing of disease presentation correlates with underlying androgen receptor (AR) transcriptomics. Synchronous metastatic disease was found to have a significantly lower median AR activity (11.8 versus 12.6; p< 0.01) and hallmark androgen response (3.2 versus 3.3; p<0.01), compared with metachronous disease. In addition, patients with synchronous disease were significantly more likely to have low AR activity (38% versus 20%; p< 0.01) and low hallmark androgen response (58% versus 38%; p< 0.01).4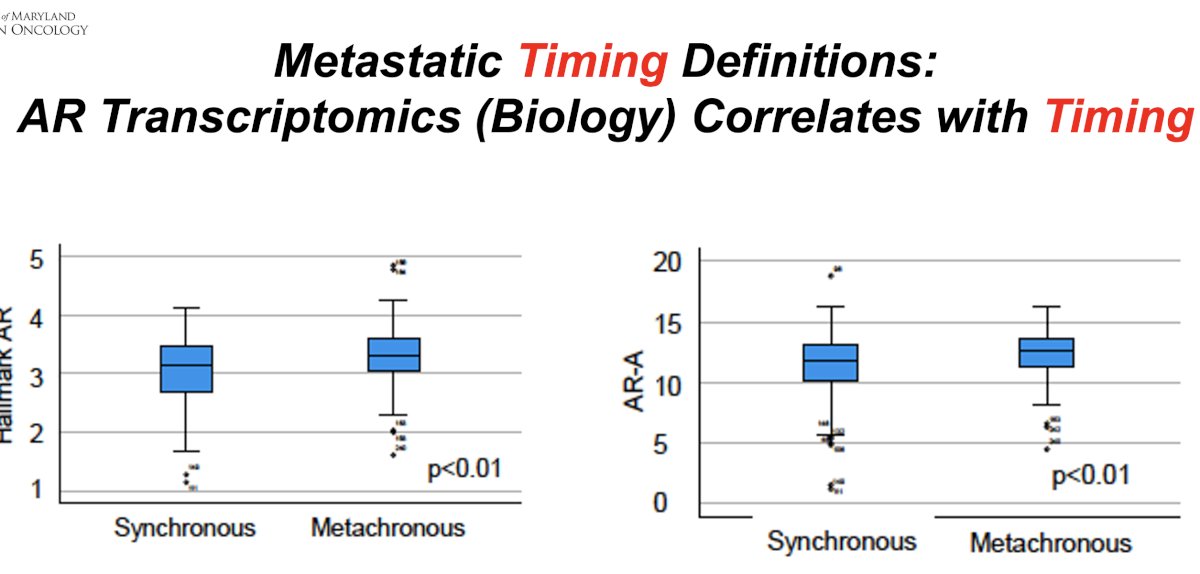
What is the evidence for MDT in oligorecurrent mCSPC patients? There are three key phase II trials in this disease space: STOMP, ORIOLE, and SABR-COMET.5-7 STOMP was a multicenter, randomized phase II trial that prospectively evaluated the effects of MDT for eugonadal men with evidence of oligometastatic disease on choline PET/CT (up to three extracranial sites) who had received prior treatment with curative intent and had evidence of biochemical recurrence. Between 2012 and 2015, 62 patients were randomized 1:1 to either surveillance or MDT, SBRT, or metastasectomy. After a median follow-up of 5.3 years, the five-year ADT-free survival was 8% in the surveillance arm compared to 34% for the MDT group (HR: 0.57, 95% CI: 0.38–0.84, log-rank p=0.06). No differences were seen between groups when stratified by nodal versus non-nodal metastases.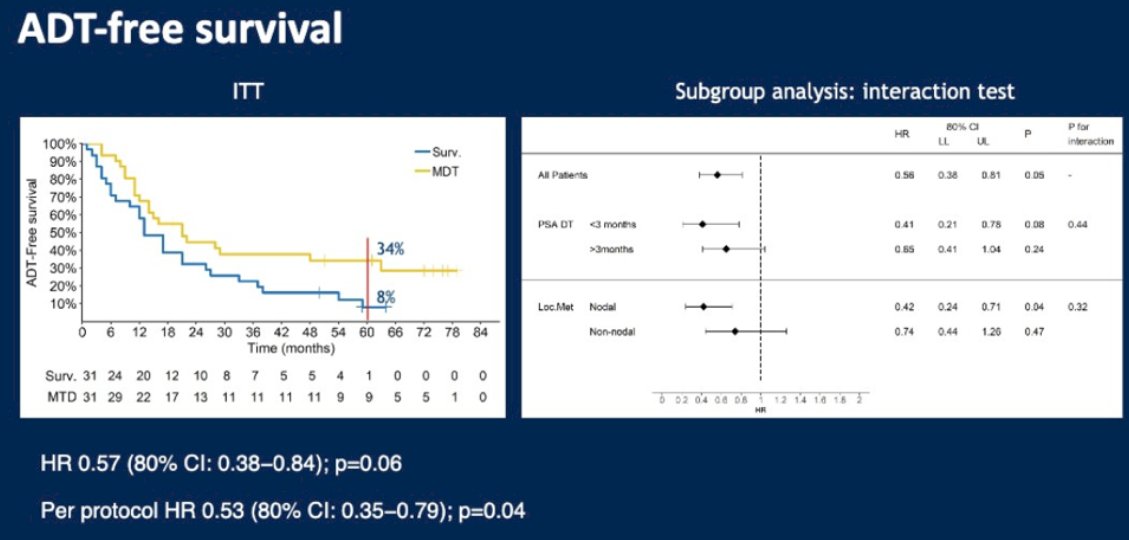
The ORIOLE trial was a randomized phase II trial of 54 men with oligorecurrent mCSPC (up to three sites). Patients were staged using conventional imaging (CT, MRI, and/or bone scan). Between 2016 and 2018, 54 men were randomized in a 2:1 fashion to receive SBRT or observation. At a median follow-up of 19 months, disease progression at six months occurred in 19% of patients in the SBRT arm versus 61% of patients in the observation arm (p=0.005).
Patients in the SBRT treatment arm had superior median progression-free survival rates (median: not reached versus 5.8 months; HR: 0.30; 95% CI: 0.11–0.81; p=0.002). Notably, no grade ≥3 treatment-related adverse events were observed with SBRT.
SABR-COMET was a randomized, open-label phase II study of patients with oligometastatic disease (up to five sites) between February 2012 and August 2016. This trial was not restricted to patients with prostate cancer and also included lung, breast, and colorectal cancer patients. Of the 99 patients in this trial, 18 (18.2%) had prostate cancer. After stratifying by the number of metastases (1–3 versus 4–5), patients were randomized in a 1:2 fashion to receive either palliative standard of care alone or standard of care plus SBRT.
At a median follow-up of 5.7 years, the primary outcome of overall survival was superior for SBRT-treated patients. The 8-year overall survivals were 27% and 14% in the intervention and control arms, respectively (HR: 0.50, 95% CI: 0.30–0.84, p=0.008). The 8-year progression-free survival estimates were 21% and 0%, respectively (HR: 0.45, 95% CI: 0.28–0.72, p<0.001).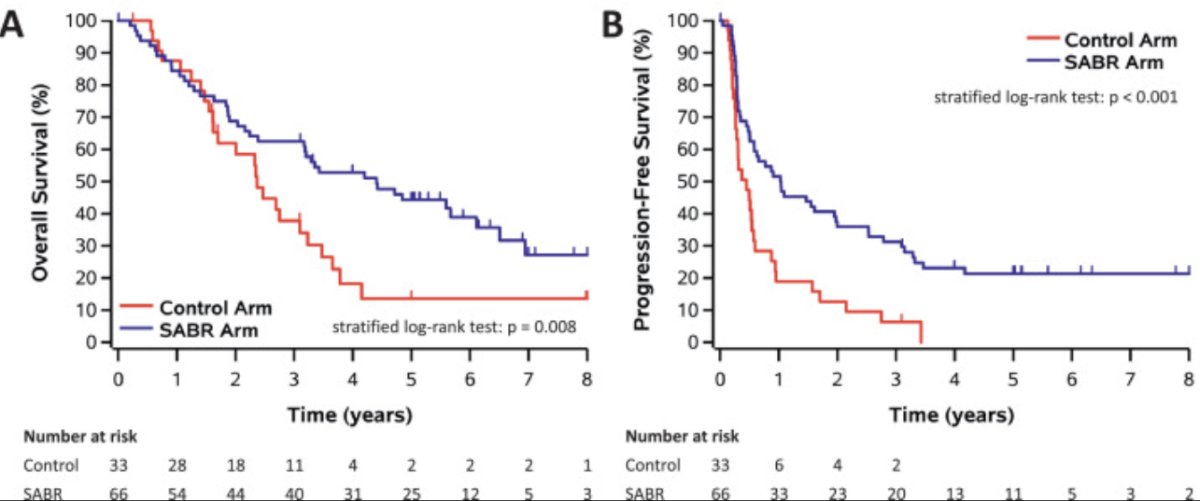
The rates of grade ≥2 acute or late toxic effects were 30% versus 9% (p=0.019), with no new grade 3 to 5 toxic effects. The FACT-G quality-of-life scores declined over time in both arms, but there were no differences in quality-of-life scores between the study arms.
Dr. Tran concluded his presentation with the following key take-home messages:
- Space and Time in mCSPC are clinical manifestations (i.e., phenotypes) that are correlated with outcome and can direct treatment
- Space and Time are actually clinical manifestations-phenotypes of underlying metastatic biology
- Understanding the underlying metastatic biology better can instruct rationale design of combination systemic and local therapy for metastatic disease.
- Better understanding of oligometastatic biology will have implications for polymetastatic disease.
Presented by: Phuoc Tran, MD, PhD, Vice Chair of Research, Professor of Radiation Oncology, University of Maryland School of Medicine, Baltimore, MD
Written by: Rashid Sayyid, MD, MSc – Robotic Urologic Oncology Fellow at The University of Southern California, @rksayyid on Twitter during the 2024 ASTRO Annual Congress held in Washington, DC between September 29th and October 2nd, 2024
References:- Parker CC, James ND, Brawley CD, et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): A randomized controlled phase 3 trial. Lancet. 2018; ;392(10162):2353-2366.
- Ali A, Hoyle A, Haran AM, et al. Association of bone metastatic burden with survival benefit from prostate radiotherapy in patients with newly diagnosed metastatic prostate cancer: A secondary analysis of a randomized clinical trial. JAMA Oncol. 2021; 7(4):555-563.
- Sutera P, Van Der Eecken K, Kishan AU, et al. Definitions of disease burden across the spectrum of metastatic castration-sensitive prostate cancer: comparison by disease outcomes and genomics. Prostate Cancer Prostatic Dis. 2022; 25(4):713-9.
- Sutera PA, Shetty AC, Hakansson A, et al. Transcriptomic and clinical heterogeneity of metastatic disease timing within metastatic castration-sensitive prostate cancer. Annal Oncol. 2023; 34(7):605-14.
- Ost P, Reynders D, Decaestecker K, et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence (STOMP): Five-year results of a randomized phase II trial. J Clin Oncol. 2020;38:6_suppl.
- Philips R, Shi WY, Deek M, et al. Outcomes of Observation vs Stereotactic Ablative Radiation for Oligometastatic Prostate Cancer: The ORIOLE Phase 2 Randomized Clinical Trial. JAMA Oncol. 2020;6(5):650-9.
- Harrow S, Palma DA, Olson R, et al. Stereotactic Radiation for the Comprehensive Treatment of Oligometastases (SABR-COMET): Extended Long-Term Outcomes. Int J Radiat Oncol Bio Phys. 2022;114(4):611-6.


