(UroToday.com) The 2024 American Society for Radiation Oncology (ASTRO) Annual Meeting held in Washington, DC between September 29th and October 2nd, 2024, was host to a clinical trials session. Dr. Nicolas Demogeot presented the results of GETUG 14, a randomized phase III trial of short-term androgen deprivation therapy (ADT) plus high-dose radiotherapy in intermediate- and high-risk localized prostate cancer patients.
Dr. Demogeot noted that high-dose radiotherapy improves local control and biochemical failure-free survival outcomes in intermediate- and high-risk prostate cancer patients. However, to date, there has been no documented overall survival benefit. In the MARCAP consortium study, the addition of short-term androgen deprivation therapy (ST-ADT) to standard-dose radiotherapy improved all clinical outcomes.1-8 Dr. Demogeot and colleagues hypothesized that adding ST-ADT to high-dose radiotherapy (80 Gy) would improve survival outcomes in patients with localized prostate cancer.
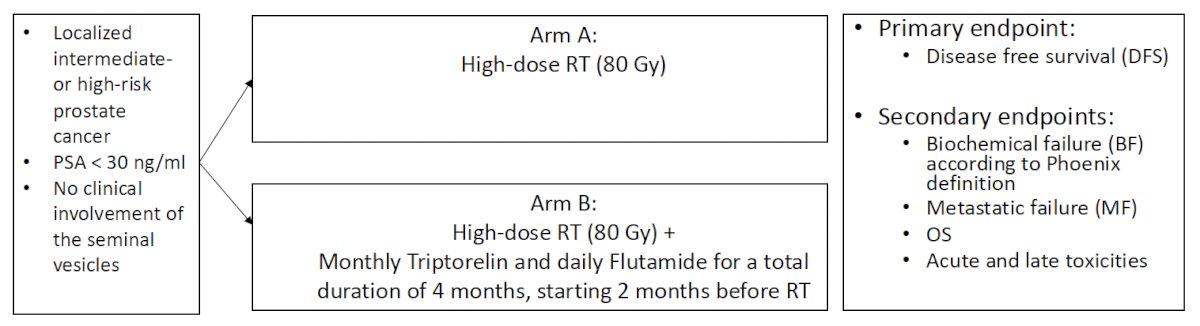
This was a multicenter, randomized phase III trial of prostate cancer patients with intermediate- or high-risk, localized prostate cancer, PSA <30 ng/ml, and without evidence of clinical seminal vesicle involvement. Eligible patients were randomized to either:
- Arm A: High-dose radiotherapy (80 Gy)
- Arm B: High-dose radiotherapy (80Gy) + monthly triptorelin + daily flutamide for a total duration of 4 months, starting 2 months prior to radiotherapy
The primary study endpoint was disease-free survival. Key secondary endpoints included:
- Biochemical failure, per the Phoenix definition
- Metastatic failure
- Overall survival
- Acute and late toxicities
Between September 2003 and June 2010, 376 patients were enrolled (Arm A: 191; Arm B: 185). In Arm B, 6 patients were not treated (5 withdrew consent; 1 experienced an acute coronary event). The modified intention-to-treat cohort thus included 179 patients in Arm B.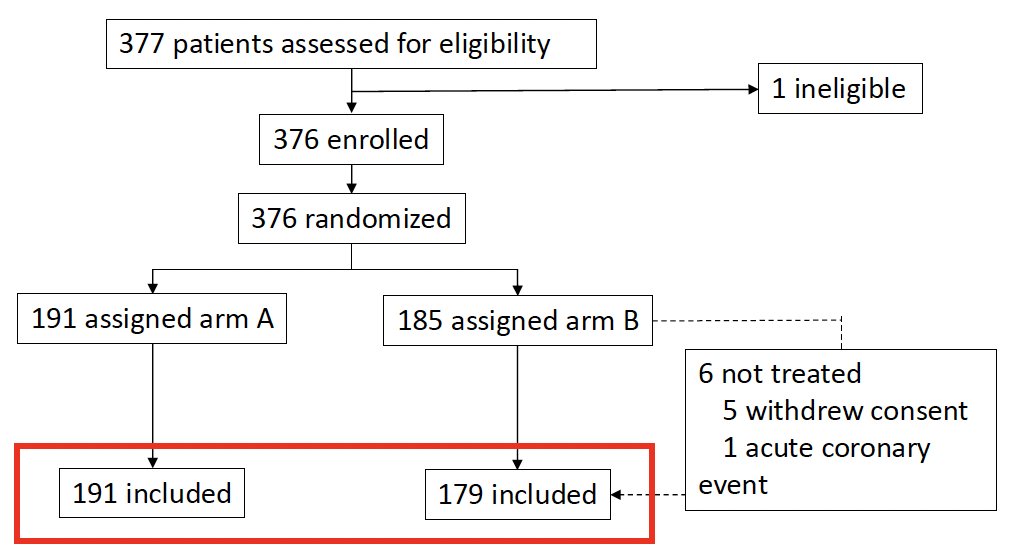
The patient characteristics are summarized below. The median patient age was 69–70 years. Overall, 12% of patients had cT3a disease (remainder ≤cT2). 40% of patients had Grade Group 3 disease. Notably ~34% had Grade Group 1 disease. 65% of patients had D’Amico intermediate-risk disease, whereas~30% had high-risk disease. 70% of patients received three-dimensional conformal radiotherapy, and 30% received intensity-modulated radiotherapy.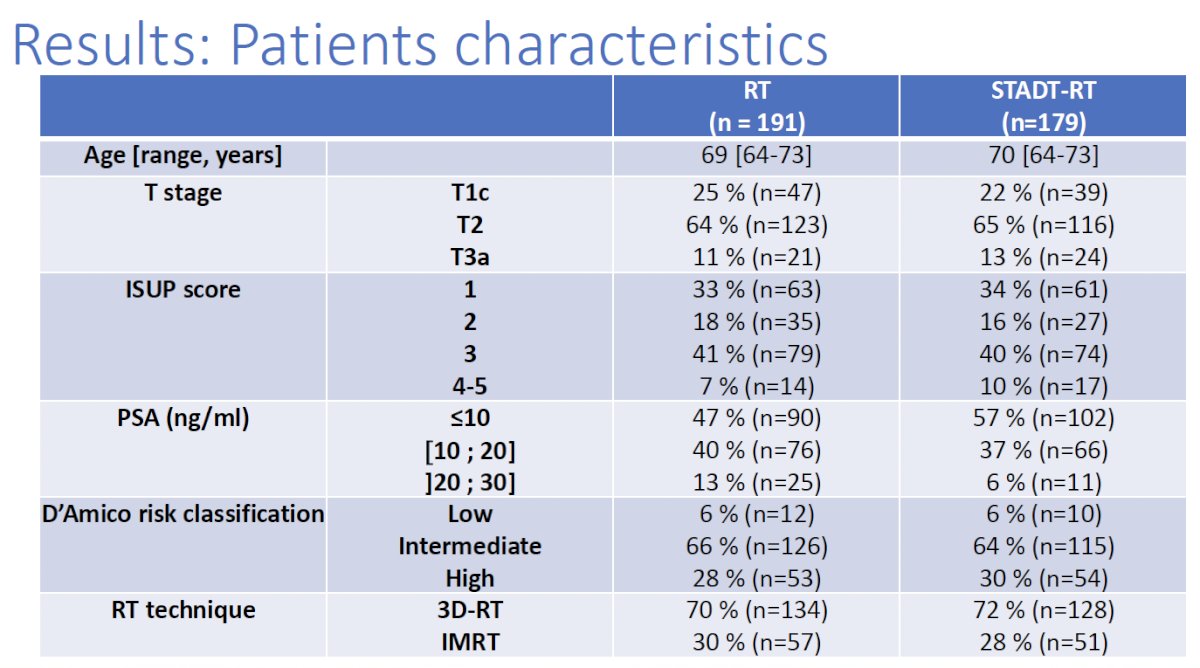
The addition of ST-ADT to radiotherapy was associated with a 36% relative improvement in disease-free survival (5-year disease-free survival: 84% versus 76%; HR: 0.64, 95% CI: 0.43–0.94, p=0.02).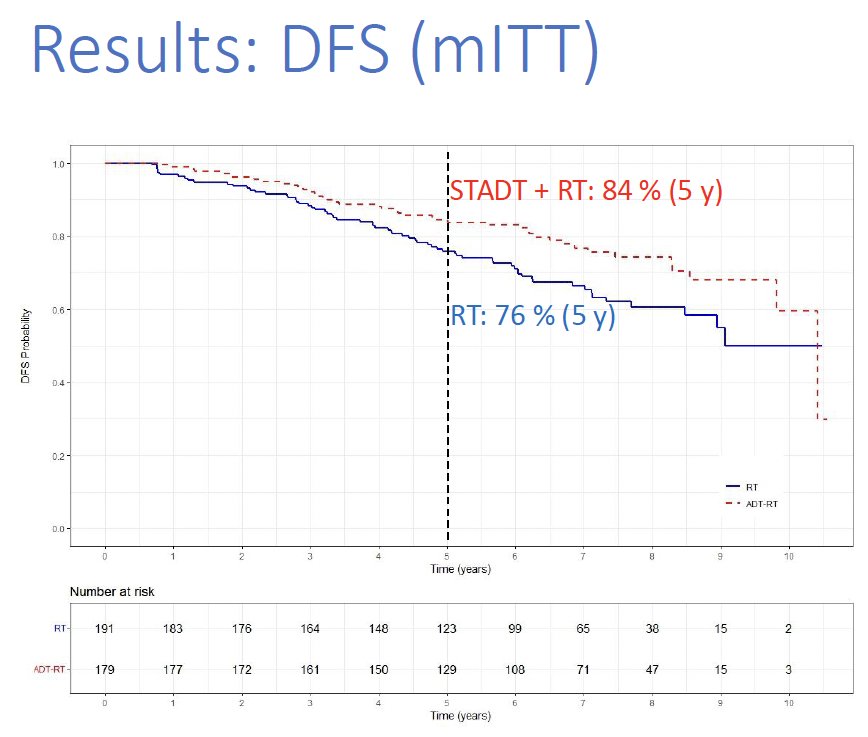
When analysis was limited to the intermediate-risk subgroup, there was a significant improvement in disease-free survival outcomes:
However, there was no significant disease-free survival benefit to the addition of ST-ADT to high-dose radiotherapy in the high-risk subgroup (HR: 0.76, p=0.40).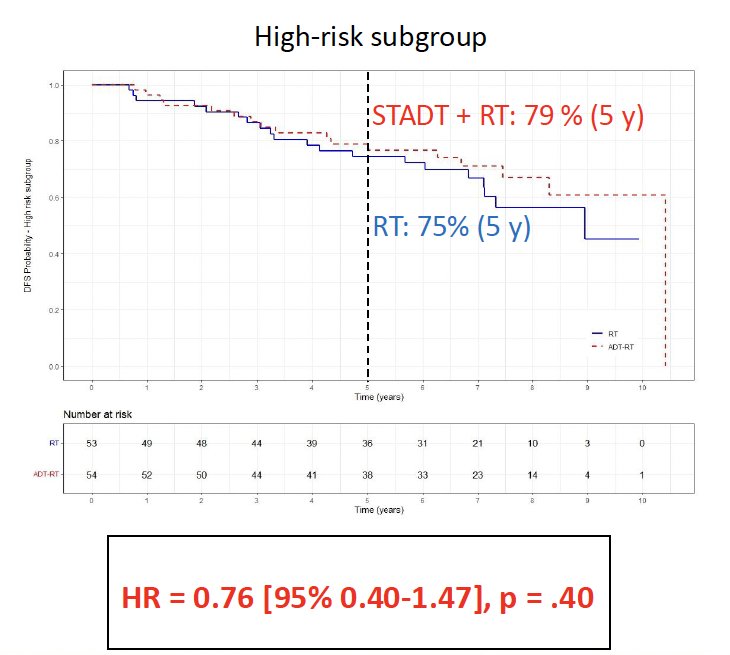
On multivariable analysis adjusted for grade and PSA level, the addition of ST-ADT to high-dose radiotherapy was found to be associated with significant disease-free survival benefits (HR: 0.66, p=0.038).
Patients who received ST-ADT were significantly less likely to experience biochemical failure (10% versus 21%; HR: 0.45, 95% CI: 0.28–0.72, p=0.001).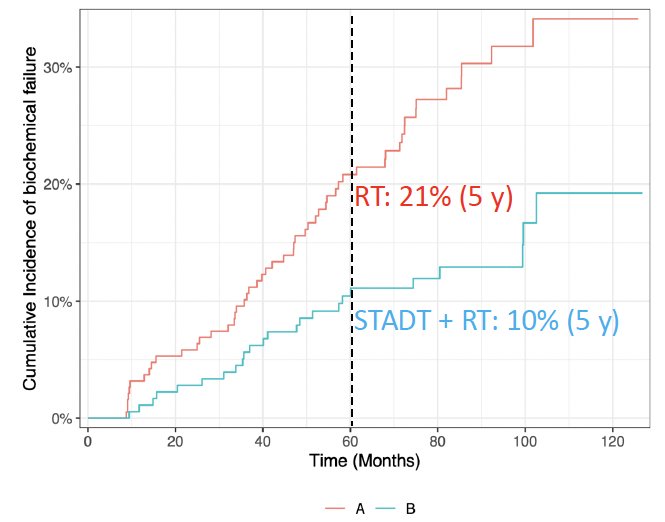
There were no significant metastasis-free or overall survival benefits.
With respect to adverse events, there were no significant differences in the proportions of patients with early or late grade ≥2 gastrointestinal or genitourinary events. Patients in the ST-ADT arm had an increased frequency of early grade ≥2 erectile dysfunction (31% versus 6%; p<0.001). There were no differences in the frequency of late grade ≥2 erectile dysfunction events.
Dr. Demogeot concluded as follows:
- Short-term ADT improves disease-free survival in intermediate- and high-risk prostate cancer patients receiving high-dose (80 Gy) radiotherapy
- Short-term ADT addition to high-dose radiotherapy does not increase the frequency of early/late genitourinary or gastrointestinal toxicities
- Limitations to this study included the following:
- Heterogenous population that included low-risk patients, in addition to intermediate- and high-risk
- Short term follow-up
- Low power for detecting differences between intermediate- and high-risk patients
- Low power for assessing metastasis-free and overall survivals
- Short-term ADT regimen was likely not long enough for high-risk patients
- Short-term ADT should be added to high-dose radiotherapy regimens
Presented by: Nicolas Demogeot, MD, Radiation Oncology, Hopitaux de Nancy, Institut Cancerologie Lorraine, Nancy, Lorraine, France
Written by: Rashid Sayyid, MD, MSc – Robotic Urologic Oncology Fellow at The University of Southern California, @rksayyid on Twitter during the 2024 ASTRO Annual Congress held in Washington, DC between September 29th and October 2nd, 2024
References:- Zelefsky MJ, Leibel SA, Gaudin PB, et al. Dose escalation with three-dimensional conformal radiation therapy affects the outcome in prostate cancer. Int J Radiat Oncol Biol Phys. 1998; 41(3):491-500.
- Dearnaley DP, Jovic G, Syndikus I, et al. Escalated-dose versus control-dose conformal radiotherapy for prostate cancer: long-term results from the MRC RT01 randomised controlled trial. Lancet Oncol. 2014; 15(4):464-73.
- Bolla M, Van Tienhoven G, Warde P, et al. External irradiation with or without long-term androgen suppression for prostate cancer with high metastatic risk: 10-year results of an EORTC randomised study. Lancet Oncol. 2010; 11(11):1066-73.
- Beckendorf V, Guerif S, Le Prise E, et al. 70 Gy versus 80 Gy in localized prostate cancer: 5-year results of GETUG 06 randomized trial. Int J Radiat Oncol Biol Phys. 2011; 80(4):1056-63.
- Hemmsbergen WD, Al-Mamgani A, Slot A, et al. Long-term results of the Dutch randomized prostate cancer trial: impact of dose-escalation on local, biochemical, clinical failure, and survival. Radiother Oncol. 2014; 110(1):104-9.
- Kuban DA, Levy LB, Cheung MR, et al. Long-term failure patterns and survival in a randomized dose-escalation trial for prostate cancer. Who dies of disease? Int J Radiat Oncol Biol Phys. 2011; 79(5):1310-7
- Zietman AL, Bae K, Slater JD, et al. Randomized trial comparing conventional-dose with high-dose conformal radiation therapy in early-stage adenocarcinoma of the prostate: long-term results from proton radiation oncology group/American College of radiology 95-09. J Clin Oncol. 2010; 28(7):1106-11.
- Ma TM, Sun Y, Malone S, et al. Sequencing of Androgen-Deprivation Therapy of Short Duration With Radiotherapy for Nonmetastatic Prostate Cancer (SANDSTORM): A Pooled Analysis of 12 Randomized Trials. J Clin Oncol. 2023; 41(4):881-92.


