(UroToday.com) The 2024 American Society for Radiation Oncology (ASTRO) annual meeting held in Washington D.C., between September 29 and October 2 was host to the session EDU 16 - Management of Unfavorable Intermediate-Risk Prostate Cancer: Role of SBRT, Brachytherapy and Androgen Deprivation Therapy. Dr. Neil Desai discussed the role of ADT and Genomic Risk Stratification for Patients with Unfavorable Intermediate-Risk Prostate Cancer.
Dr. Desai began by presenting the case of a 62-year-old gentleman with an elevated PSA of 6 ng/mL, a normal digital rectal examination (cT1c), who underwent a prostate biopsy. His biopsy revealed grade group 3 prostate cancer in 3 out of 12 cores, and he was deemed to have unfavorable intermediate-risk prostate cancer. According to the NCCN/D’Amico risk stratification, this patient could be treated with observation or radiation therapy (RT) and androgen deprivation therapy (ADT) for 4 to 6 months. This remains a challenging scenario, and patients and clinicians often struggle with decision-making due to conflicting opinions and competitive pressures. Determining whether to administer ADT and deciding on the modality and intensity of RT, can be difficult.
There are various risk-stratification tools available, such as CAPRA, STAR-CAP, different imaging modalities, and even genomic or artificial intelligence classifiers, to assess the patient's risk. We often consider patient-specific variables such as the baseline quality of life, and comorbidities, and aim to optimize the patient before treatment. We are now adding genomic/AI classifiers, and we have competition pressures, so we need to integrate these tools into our clinical practice.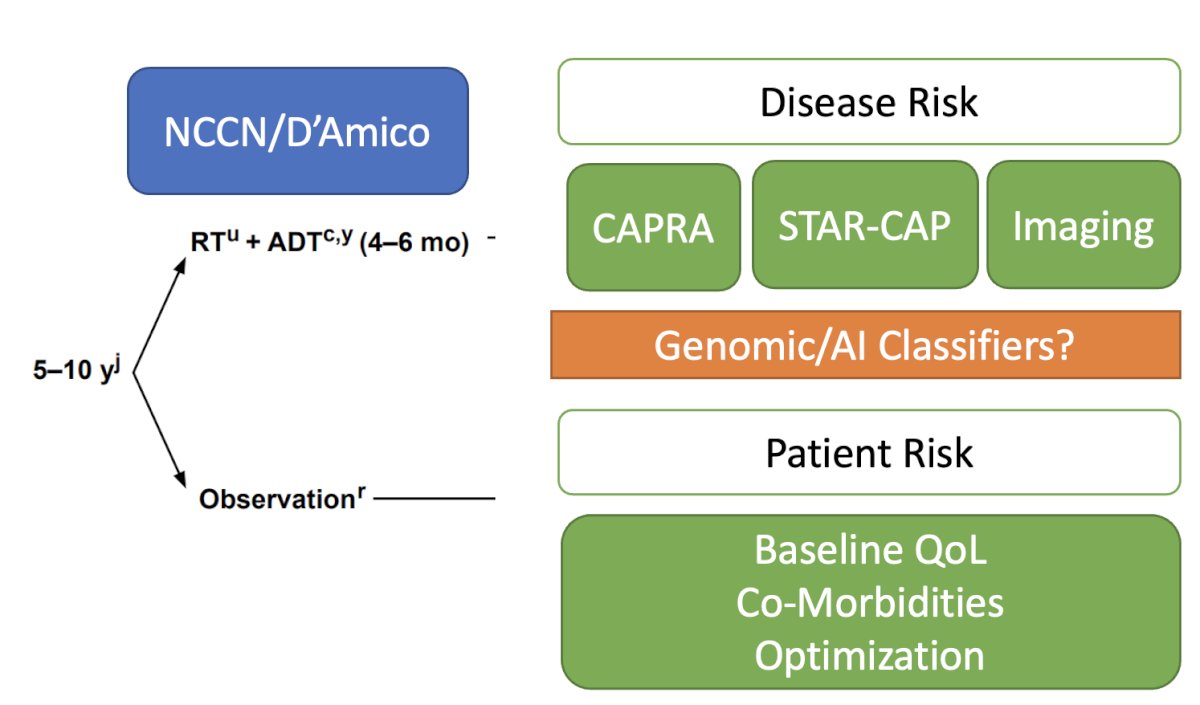
The NRG Oncology/Radiation Therapy Oncology Group (RTOG) 0815 study randomly assigned 1,492 patients with intermediate-risk prostate cancer to dose-escalated RT alone versus dose-escalated RT with short-term ADT (6 months). For this trial, RT modalities included external-beam RT alone to 79.2 Gy or external beam RT (45 Gy) with a brachytherapy boost, with a median follow-up of 6.3 years. Short-term ADT resulted in significantly reduced PSA failure, PCSM, and distant metastasis rates. However, this trial did not improve OS rates but did show an improvement in metastasis-free survival, which is often used as a surrogate endpoint for OS.1 Based on this data, we used short-term ADT in patients treated with radiation and unfavorable intermediate-risk prostate cancer.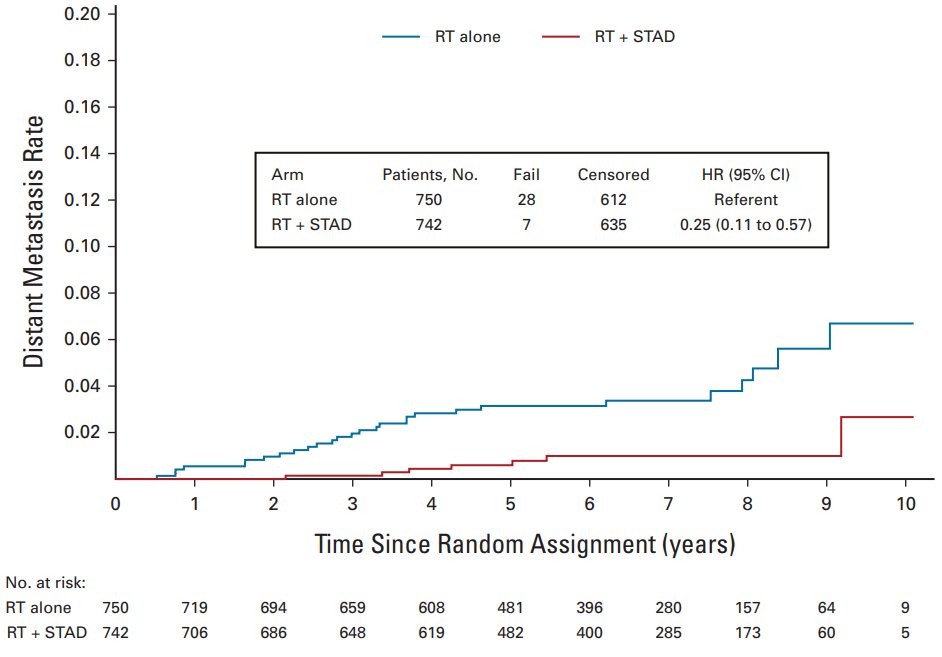
Dr. Desai polled the audience if they use advanced risk classifiers to inform ADT use in unfavorable intermediate-risk prostate cancer. The majority of the auditorium (46%) answered they don’t use these tools.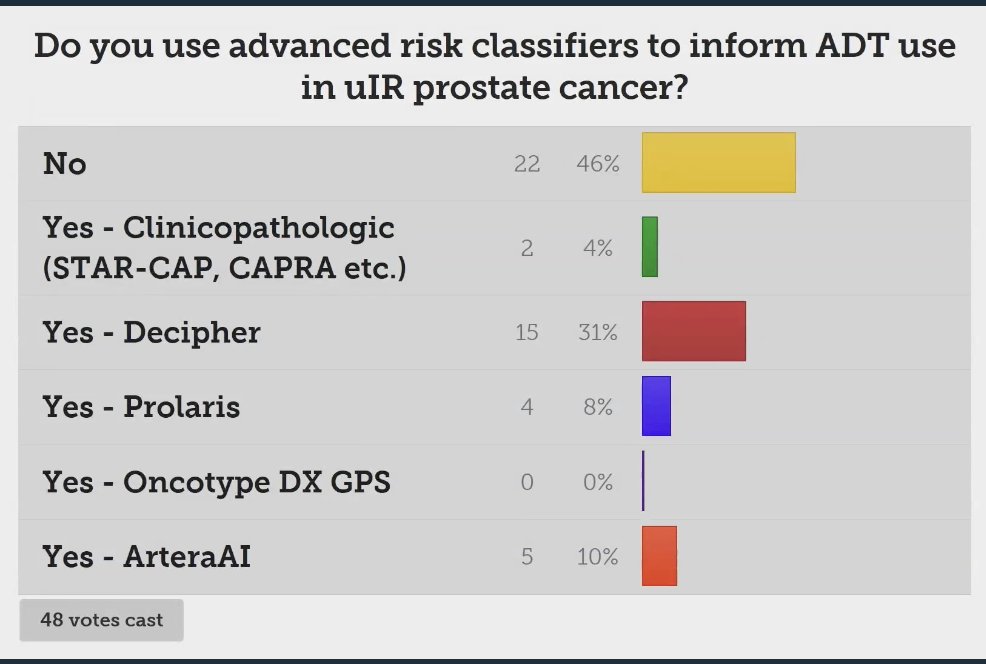
We don’t have reliable clinical risk factors to predict metastasis. Dr. Desai discussed that tossing a coin gives an area under the curve (AUC) of 0.5, which is not much different from the PSA (~0.6), clinical stage (~0.6), Gleason score (~0.6), NCCN risk stratification (~0.6), or CAPRA risk score (~0.6). For the past decade, we have been basing decisions on whether to add ADT to RT on these risk factors for patients with favorable and unfavorable intermediate-risk prostate cancer.
As prostate cancer treatment advances, we face more options, which can lead to more complex decision-making. For a patient with prostate cancer, we now have to choose among conventionally fractionated RT (CFRT), moderately hypofractionated radiotherapy (MHRT), stereotactic body radiotherapy (SBRT), micro-boost, brachytherapy boost, and proton radiotherapy. Additionally, we must decide the duration of ADT (short vs. long), consider intensification with androgen receptor pathway inhibitors (ARPIs), and determine whether to include PET/CT PSMA in our diagnostic algorithm to guide treatment. There is a lot of nuance from patient to patient, which requires a careful assessment of absolute risks and calls for personalized treatment plans.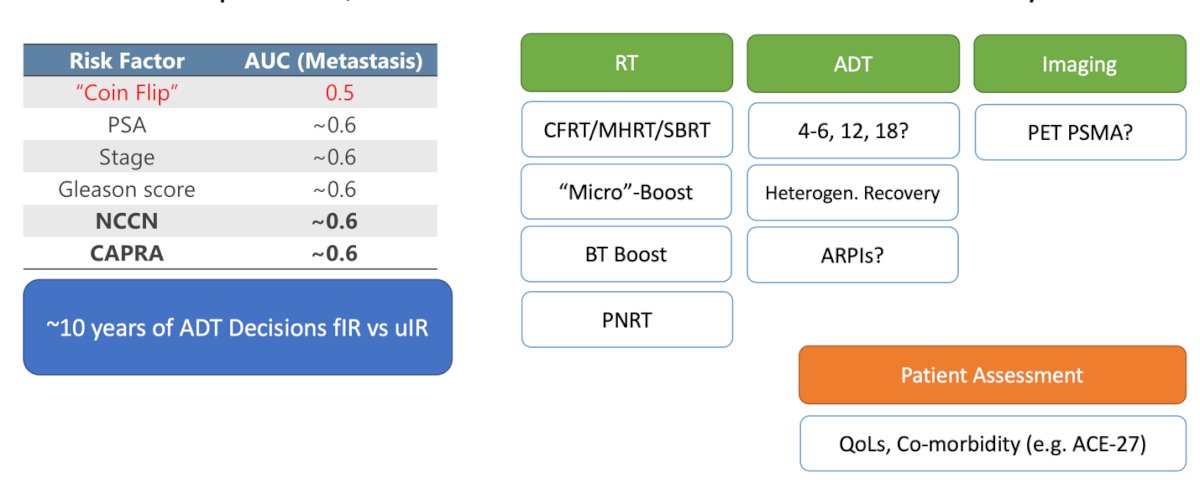
Dr. Desai engaged the audience with a poll regarding what they deemed a significant reduction in the absolute risk of 5-year metastasis through short-term androgen deprivation therapy (ADT). The results revealed that 66% of participants considered a reduction of greater than 5% to be worthwhile, while nearly a third believed that a reduction of more than 10% constituted a meaningful benefit.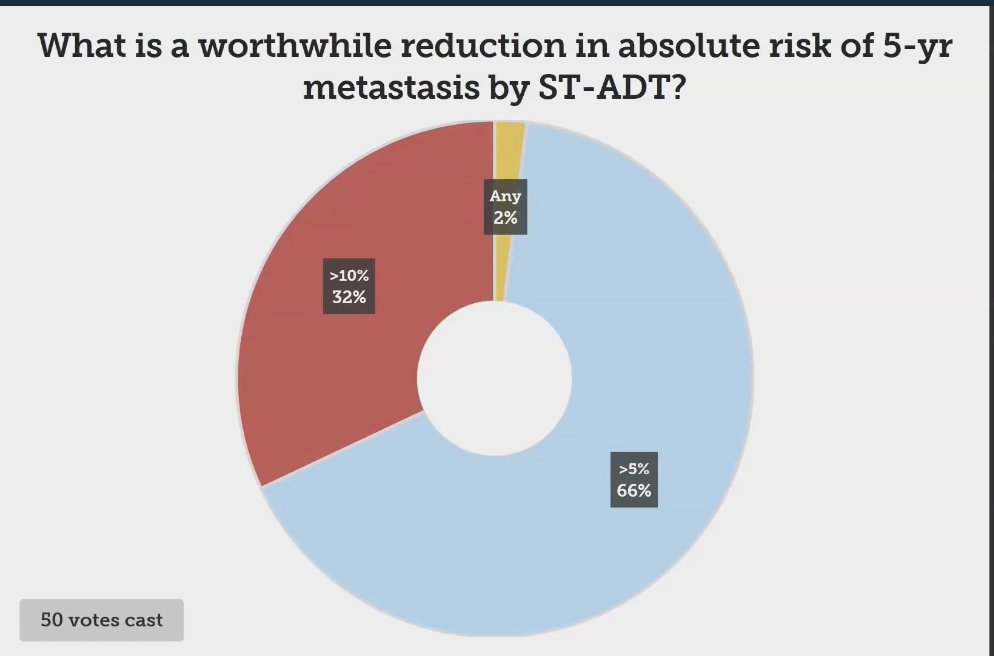
Different tools can be used as advanced risk classifiers for prostate cancer. These include mRNA classifiers such as Decipher, Prolaris, and Oncotype DX GPS. Another exciting, emerging tool is multi-modal artificial intelligence (MMAI) classifiers.
Prolaris is a panel of cell cycle progression (CCP) genes measuring cancer proliferation. The primary outcome of interest for this risk classifier is the 10-year risk of prostate cancer mortality. The Oncotype DX prostate biopsy test calculates a genomic prostate score (GPS) based on genes from four different pathways involved in prostate cancer. The Decipher test is a clinical-grade high-density microarray that detects RNA expression levels, using 22 RNA expression-based genomic markers prognostic for metastatic progression.
The genomic signatures for all these tests have been developed and validated, with most evidence to date describing the Genomic Classifier signature that predicts metastatic progression of localized disease. Shockingly, there was minimal overlap in signatures and development of these mRNA classifiers. The NCCN recommends the Genomic Classifier (Decipher) to guide treatment, and the CAPRA-CCP score has been validated with Prolaris. The signatures are outlined in the figure below: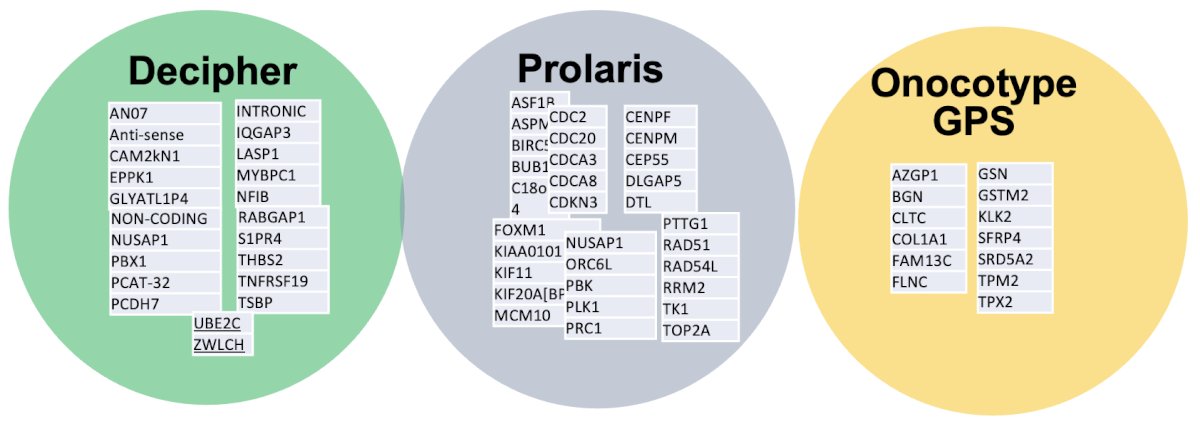
The NCCN principle of risk-stratification using advanced tools for localized prostate cancer, describes in detail, each one of this genomic classifiers.
The MMAI prognostic model was developed using pathology images, NCCN variables (combined Gleason score, T-stage, baseline PSA), age, Gleason primary, and Gleason secondary scores, which were assembled from a unique dataset comprising five large multinational randomized phase III clinical trials of men with localized prostate cancer (NRG/RTOG-9202, 9408, 9413, 9910, and 0126). All patients received definitive external RT with or without the pre-specified use of ADT. Data from all five clinical trials were split into training (80%) and validation (20%) sets.2
The MMAI model consistently outperformed the NCCN risk groups across all tested outcomes, including distant metastasis at 5 and 10 years, biochemical failure at 5 and 10 years, prostate cancer-specific survival, and overall survival, when comparing the performance results for the validation set.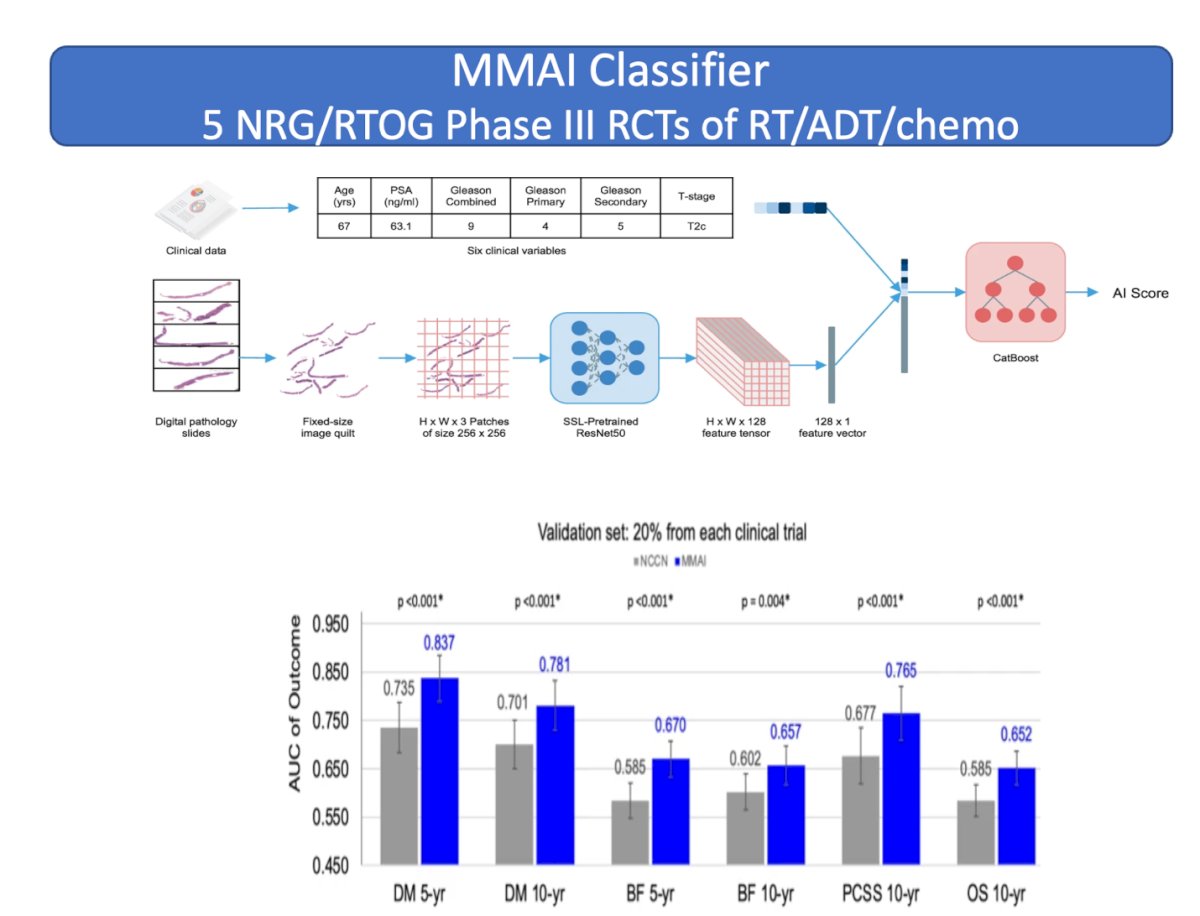
We have advanced risk classifiers including three RNA expression genomic signatures (Decipher, Prolaris, Oncotype DX GPS) and the MMAI prognostic model. All of these classifiers have different prognostic endpoints and varying levels of evidence, but they have generally improved prognostication, especially for distant metastasis, achieving an AUC of around 0.8. This is significantly superior to the other risk factors mentioned earlier.
The Decipher GC signature has a Simon level of evidence IB for guiding the addition of short-term ADT to RT in intermediate-risk prostate cancer. This is based on the NRG/RTOG 0126 phase III randomized trial post-hoc analysis, which demonstrated the independent prognostic effect of GC on biochemical failure, secondary therapy, distant metastasis (DM), prostate cancer-specific mortality (PCSM), metastasis-free survival (MFS), and overall survival (OS). Patients receiving RT alone with low GC scores had 10-year DM rates of 4%, compared with 16% for those with high GC risk. Therefore, the NCCN guidelines recommend that RT alone should be considered for patients with a low GC score and NCCN intermediate-risk disease. The addition of short-term ADT should be considered for patients with a high GC score, given their increased risk of DM and the significant benefit of short-term ADT on DM, even with dose-escalated RT or brachytherapy boost.3
MMAI models also have a Simon-level IB as predictive biomarkers to estimate the benefit of short-term ADT with radiotherapy and long-term ADT with radiotherapy. Additionally, they serve as prognostic biomarkers to estimate the risk for distant metastasis and death from prostate cancer.2,4,5 The principles of advanced risk stratification as summarized by the NCCN guidelines are outlined below: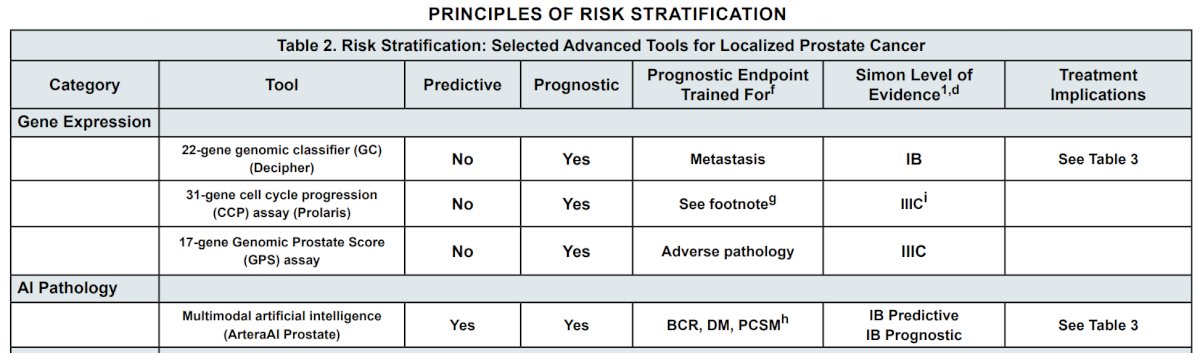
Dr Desai mentioned he sees two major applications of mRNA classifiers for patients with intermediate-risk prostate cancer:
- De-intensification by omission of short-term ADT with RT
- Intensification of ADT with RT
- De-intensification by omission of short-term ADT with RT
Lower Decipher GC and Prolaris CCP scores in patients with intermediate-risk prostate cancer demonstrate low distant metastasis risks with RT alone, raising the question of whether it is necessary to add ADT to RT in these patients. Dr. Desai mentioned that a low Prolaris score is associated with a 3-4% risk of distant metastasis, and a low Decipher score has shown that RT alone might be sufficient in an ongoing randomized controlled trial.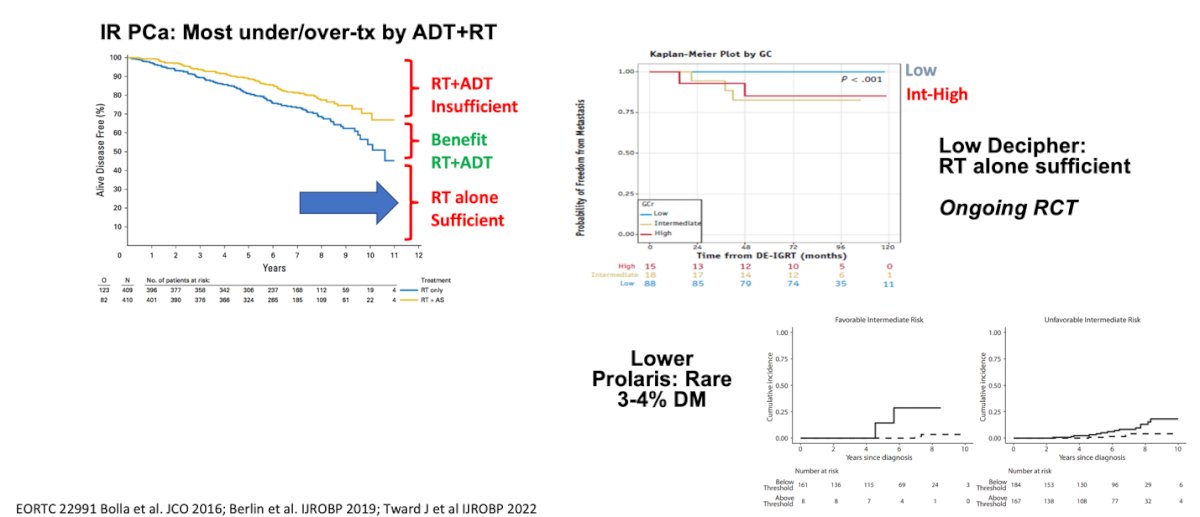
Intermediate Risk Prostate Cancer Intensification of ADT with RT
Patients with unfavorable intermediate-risk prostate cancer and Decipher GC intermediate/high-risk scores have a higher risk of distant metastasis and often behave like high-risk prostate cancer. There is mixed data on the benefit of extending ADT from 6 months (short-term) to 18 months (long-term), but mostly no one uses long-term ADT in unfavorable intermediate-risk prostate cancer nowadays. This approach requires novel intensification strategies or better patient selection.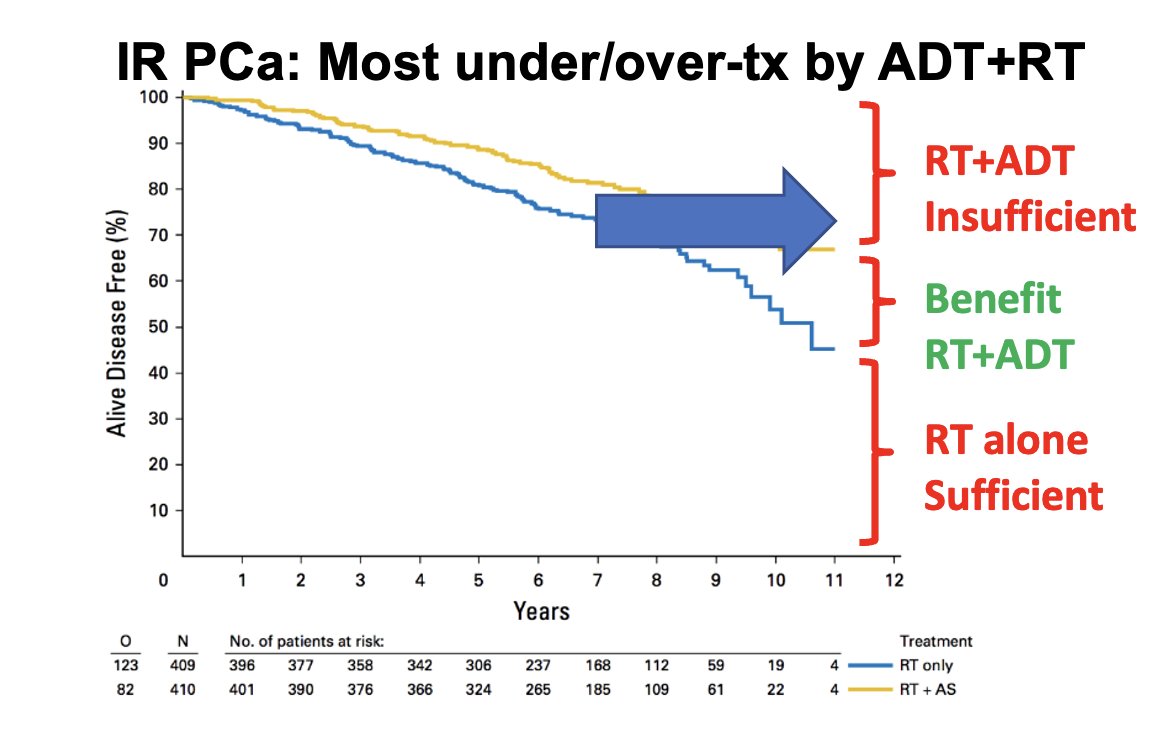
Dr. Desai discussed the TROG 03.04 RADAR trial, which explored 6 or 18 months of ADT in addition to 66, 70, or 74 Gy of external beam radiation therapy (EBRT) or 46 Gy of EBRT plus high-dose-rate brachytherapy boost (HDRB). Almost a third of the patients in this trial had unfavorable intermediate-risk prostate cancer. The trial showed that 18 months of ADT significantly improved metastasis-free survival and biochemical progression-free survival as illustrated below.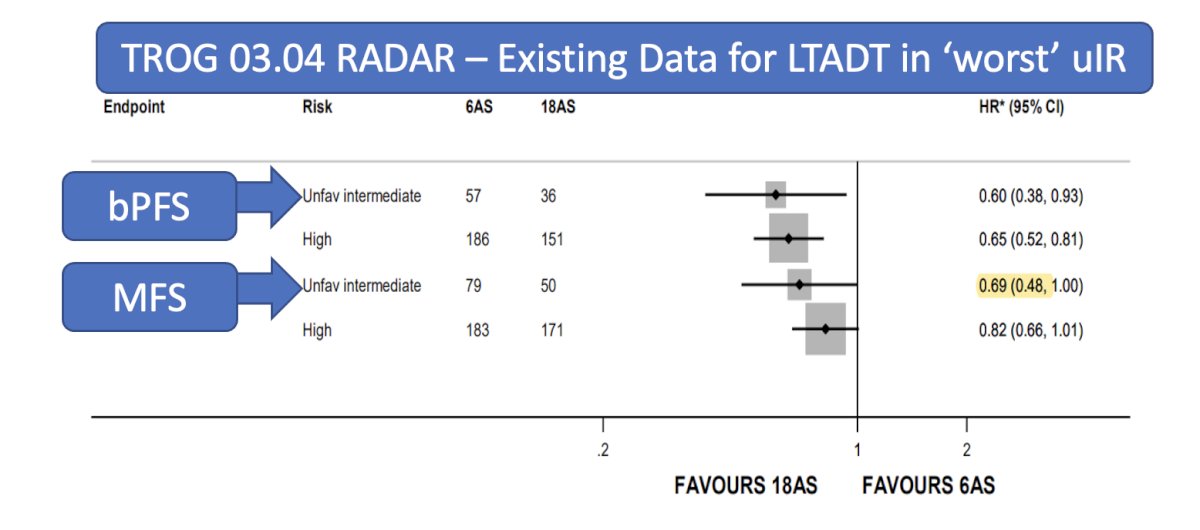
There is an ongoing randomized controlled trial, the NRG-GU010: GUIDANCE study, Parallel phase III randomized trials of genomic-risk stratified unfavorable intermediate-risk prostate cancer to inform de-intensification and intensification (NCT05050084). Briefly, this study uses the Decipher score (<0.4 or ≥ 0.4) to randomize patients to RT alone, RT with short-term ADT, or RT with short-term ADT plus darolutamide. The study design is outlined below. This trial aims to shed light on this important clinical dilemma.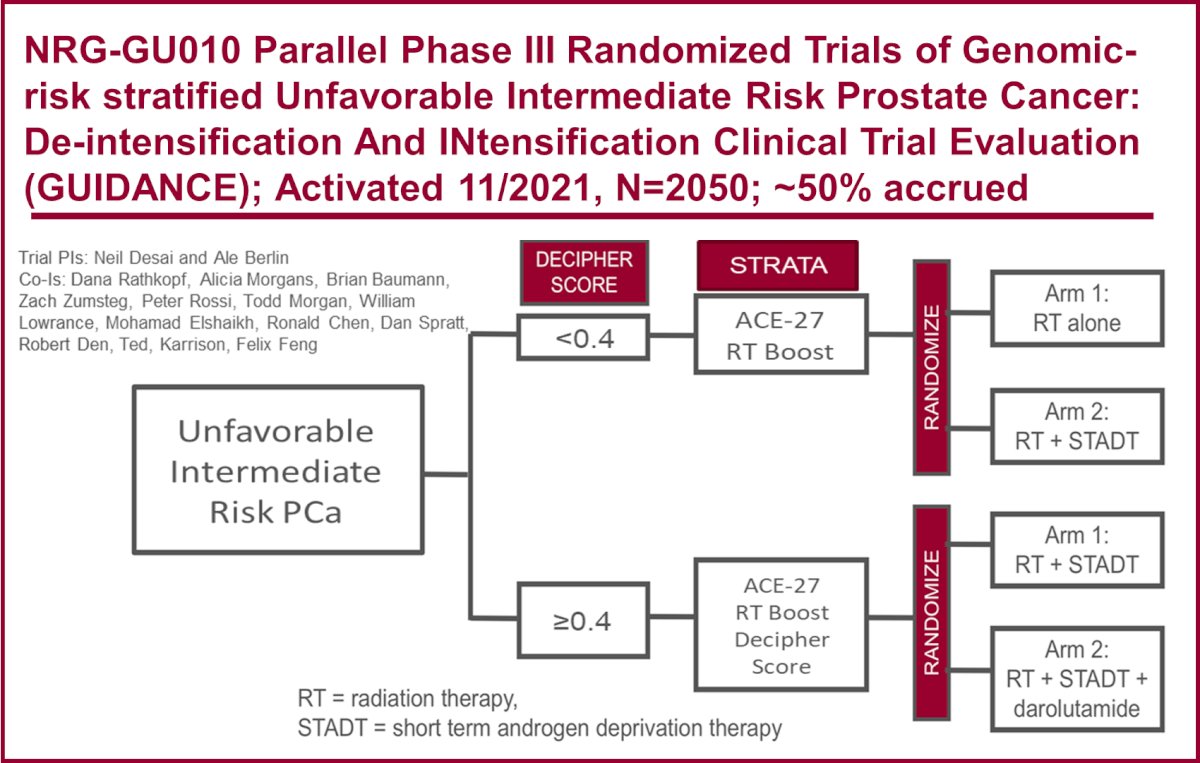
All the previous biomarkers are prognostic biomarkers, and we are looking for predictive biomarkers. MMAI has predictive value for determining the benefit of adding short-term ADT to radiotherapy for intermediate-risk prostate cancer patients. The MMAI model was trained using data from the phase III NRG/RTOG randomized clinical trials 9202, 9413, 9910, and 0126, using a 67th percentile cutoff for positive versus negative results. The MMAI was validated in the NRG/RTOG 9408 trial in patients with intermediate-risk prostate cancer to assess its ability to inform the addition of short-term ADT to RT.4
The data unequivocally showed that using the MMAI model significantly informed the decision to use short-term ADT. Patients with a positive MMAI predictive model who received short-term ADT had a significantly lower 15-year risk of distant metastasis and prostate cancer-specific mortality.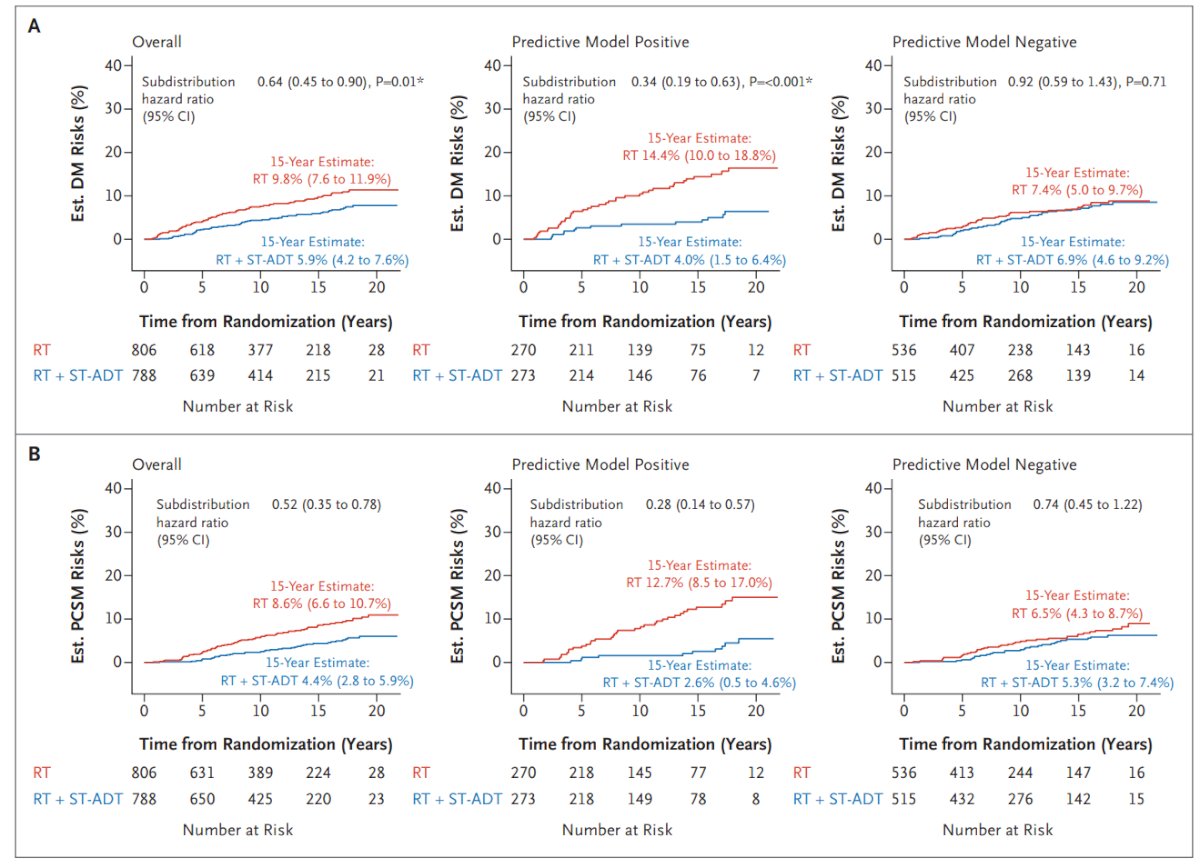
Guiding RT intensity
RT dose intensification trades cancer control versus toxicity and benefits only a minority of patients. There is emerging Data for Classifier prognostication and prediction of radiation therapy intensity.
Using the predictive performance of a 24-gene post-operative radiation therapy outcomes score (PORTOS) in the SAKK 09/10 phase III randomized trial of dose-intensified salvage radiotherapy after radical prostatectomy (64 Gy vs. 70 Gy) to the prostatic fossa, the PORTOS score was found to be predictive of response to higher radiation doses. Patients in the higher PORTOS group exhibited significantly improved clinical progression-free survival rates with dose escalation to 70 Gy compared to 64 Gy (HR: 0.19, 95% CI: 0.05 – 0.70, p=0.01).7
Similarly, in an analysis of the RTOG 0126 trial (CFRT 70.2 Gy vs. 79 Gy), there was a significant interaction based on Decipher low versus high-risk scores, particularly in patients with high-risk profiles regarding distant metastasis-free survival. Notably, patients with lower risk did not benefit from the intensification of radiation therapy.8
Dr. Desai concluded his presentation with the following key takeaways:
- Management of unfavorable intermediate-risk prostate cancer is becoming increasingly heterogeneous, both in terms of the patient population and the management options available.
- Advanced risk classifiers serve as critical “level setters” empowering patients with actionable information to facilitate more informed clinical decision-making.
- Resulting serial visits can be used to solidify discussions on ADT/RT with our patients.
- Engagement with pathology and vendors is essential for ensuring efficient, collegial workflows and mainly regarding on how to reduce financial toxicity for patients.
Presented by: Neil Desai, MD, Assistant Professor of Radiation Oncology at University of Texas Southwestern Medical Center, Dallas, TX.
Written by: Julian Chavarriaga, MD – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @chavarriagaj on Twitter during the 2024 American Society for Radiation Oncology (ASTRO) annual meeting held in Washington D.C., between the 29th of September and the 2nd of October.
References:- Krauss DJ, Karrison T, Martinez AA, Morton G, Yan D, Bruner DW, Movsas B, Elshaikh M, Citrin D, Hershatter B, Michalski JM, Efstathiou JA, Currey A, Kavadi VS, Cury FL, Lock M, Raben A, Seaward SA, El-Gayed A, Rodgers JP, Sandler HM. Dose-Escalated Radiotherapy Alone or in Combination With Short-Term Androgen Deprivation for Intermediate-Risk Prostate Cancer: Results of a Phase III Multi-Institutional Trial. J Clin Oncol. 2023 Jun 10;41(17):3203-3216. doi: 10.1200/JCO.22.02390. Epub 2023 Apr 27. PMID: 37104748; PMCID: PMC10489479.
- Esteva A, Feng J, van der Wal D, Huang SC, Simko JP, DeVries S, Chen E, Schaeffer EM, Morgan TM, Sun Y, Ghorbani A, Naik N, Nathawani D, Socher R, Michalski JM, Roach M 3rd, Pisansky TM, Monson JM, Naz F, Wallace J, Ferguson MJ, Bahary JP, Zou J, Lungren M, Yeung S, Ross AE; NRG Prostate Cancer AI Consortium; Sandler HM, Tran PT, Spratt DE, Pugh S, Feng FY, Mohamad O. Prostate cancer therapy personalization via multi-modal deep learning on randomized phase III clinical trials. NPJ Digit Med. 2022 Jun 8;5(1):71. doi: 10.1038/s41746-022-00613-w. Erratum in: NPJ Digit Med. 2023 Feb 22;6(1):27. doi: 10.1038/s41746-023-00769-z. PMID: 35676445; PMCID: PMC9177850.
- Spratt DE, Liu VYT, Michalski J, Davicioni E, Berlin A, Simko JP, Efstathiou JA, Tran PT, Sandler HM, Hall WA, Thompson DJS, Parliament MB, Dayes IS, Correa RJM, Robertson JM, Gore EM, Doncals DE, Vigneault E, Souhami L, Karrison TG, Feng FY. Genomic Classifier Performance in Intermediate-Risk Prostate Cancer: Results From NRG Oncology/RTOG 0126 Randomized Phase 3 Trial. Int J Radiat Oncol Biol Phys. 2023 Oct 1;117(2):370-377. doi: 10.1016/j.ijrobp.2023.04.010. Epub 2023 May 2. Erratum in: Int J Radiat Oncol Biol Phys. 2024 Sep 23:S0360-3016(24)03323-6. doi: 10.1016/j.ijrobp.2024.08.044. PMID: 37137444; PMCID: PMC10949135.
- Spratt DE, Tang S, Sun Y, et al. Artificial Intelligence Predictive Model for Hormone Therapy Use in Prostate Cancer. NEJM Evid 2023;2(8).
- Armstrong A, et al. Development and validation of an AI-derived digital pathology-based biomarker to predict benefit of LT-ADT with RT in men with localized high-risk prostate cancer across multiple phase III NRG/RTOG trials. ASCO 2023.
- Joseph D, Denham JW, Steigler A, Lamb DS, Spry NA, Stanley J, Shannon T, Duchesne G, Atkinson C, Matthews JHL, Turner S, Kenny L, Christie D, Tai KH, Gogna NK, Kearvell R, Murray J, Ebert MA, Haworth A, Delahunt B, Oldmeadow C, Attia J. Radiation Dose Escalation or Longer Androgen Suppression to Prevent Distant Progression in Men With Locally Advanced Prostate Cancer: 10-Year Data From the TROG 03.04 RADAR Trial. Int J Radiat Oncol Biol Phys. 2020 Mar 15;106(4):693-702. doi: 10.1016/j.ijrobp.2019.11.415. PMID: 32092343.
- Dal Pra, A. et al.Validation of the Decipher genomic classifier in patients receiving salvage radiotherapy without hormone therapy after radical prostatectomy – an ancillary study of the SAKK 09/10 randomized clinical trial☆ Annals of Oncology, Volume 33, Issue 9, 950 - 958
- Spratt DE, Liu VYT, Michalski J, Davicioni E, Berlin A, Simko JP, Efstathiou JA, Tran PT, Sandler HM, Hall WA, Thompson DJS, Parliament MB, Dayes IS, Correa RJM, Robertson JM, Gore EM, Doncals DE, Vigneault E, Souhami L, Karrison TG, Feng FY. Genomic Classifier Performance in Intermediate-Risk Prostate Cancer: Results From NRG Oncology/RTOG 0126 Randomized Phase 3 Trial. Int J Radiat Oncol Biol Phys. 2023 Oct 1;117(2):370-377. doi: 10.1016/j.ijrobp.2023.04.010. Epub 2023 May 2. Erratum in: Int J Radiat Oncol Biol Phys. 2024 Sep 23:S0360-3016(24)03323-6. doi: 10.1016/j.ijrobp.2024.08.044. PMID: 37137444; PMCID: PMC10949135.


