(UroToday.com) The 2024 American Society for Radiation Oncology (ASTRO) annual meeting held in Washington D.C., between September 29 and October 2 was host to the session EDU 16 - Management of Unfavorable Intermediate-Risk Prostate Cancer: Role of SBRT, Brachytherapy and Androgen Deprivation Therapy. Dr. Daniel Spratt discussed SBRT for Unfavorable Intermediate-Risk Prostate Cancer.
Dr. Spratt began his presentation by noting that when we think about risk stratification in prostate cancer, the first things that come to mind are the NCCN risk categories or D’Amico risk stratification. However, the classification of intermediate-risk prostate cancer into favorable and unfavorable categories is relatively new. This division first occurred in 2013. Dr. Spratt mentioned that it all started with Dr. Desai in the residents' room, where they discussed the observation that some patients within this risk group exhibit more aggressive behavior. This led to the proposal of the new classification, which serves as a prognostic factor.
Unfavorable intermediate-risk prostate cancer is often associated with a poor prognosis. As shown below, having Gleason grade group 3 (HR 3.49) or ≥2 intermediate risk factors (HR 2.40) are associated with an increased risk of distant metastasis. Similarly, having unfavorable intermediate-risk prostate cancer is associated with an increased cumulative incidence of prostate cancer-specific mortality compared to favorable intermediate-risk disease (p=0.013).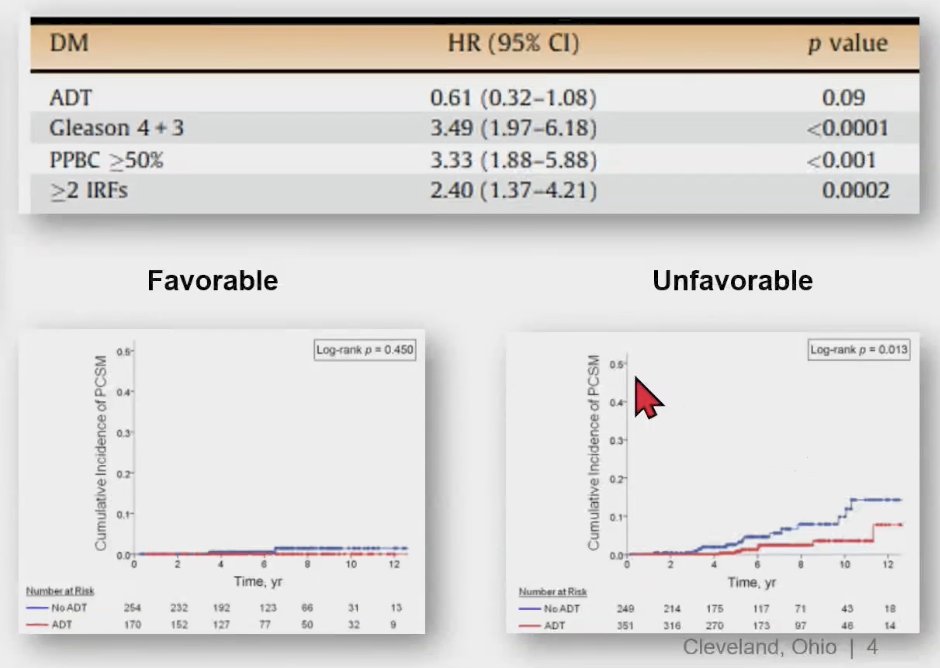
These findings were confirmed through a secondary analysis of NRG Oncology's RTOG 9408 (NCT00002597), a randomized clinical trial of 1,068 intermediate-risk prostate cancer patients who were treated with radiotherapy with or without 4 months of androgen deprivation therapy (ADT). Patients with unfavorable intermediate-risk had a higher risk of distant metastasis (HR 2.36; 95% CI, 1.44 to 3.89; P = .001) and prostate cancer-specific mortality (HR, 1.84; 95% CI, 1.29 to 2.62; P = .001). Furthermore, in patients with favorable intermediate-risk disease, ADT did not improve distant metastasis or prostate cancer mortality. However, in patients with UIR, ADT improved distant metastasis (HR, 0.48; 95% CI, 0.28 to 0.83; P = .008) and prostate cancer-specific mortality (HR, 0.40; 95% CI, 0.26 to 0.60; P < .001), as shown in the curves below.1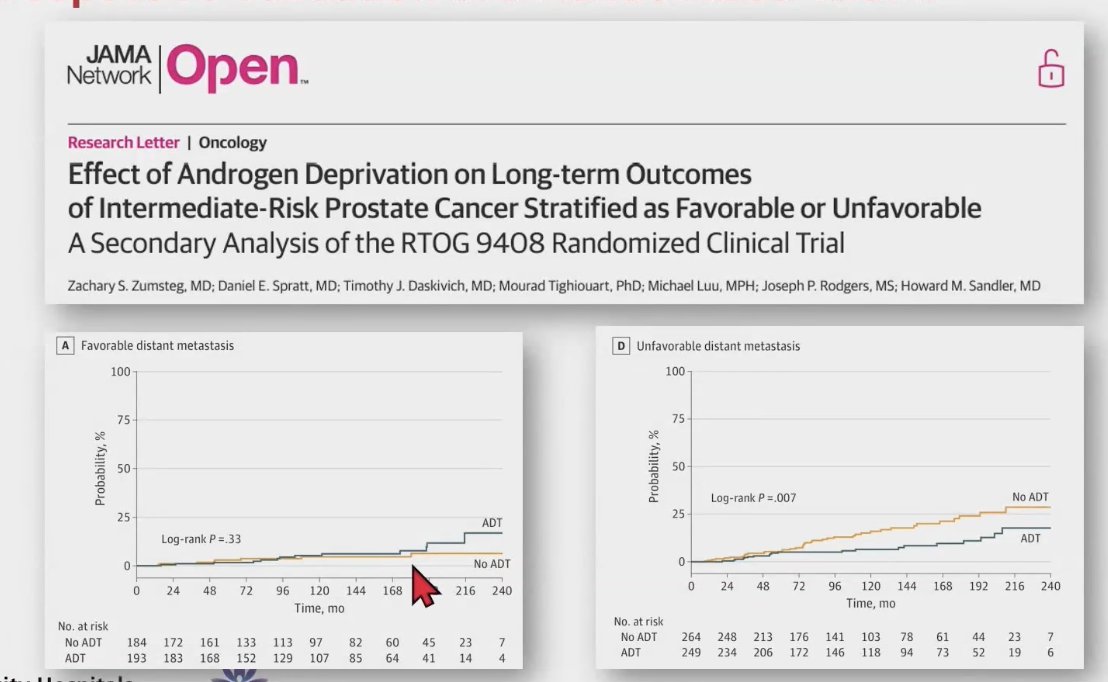
Currently, there are numerous radiotherapy treatment options for patients with unfavorable intermediate-risk prostate cancer, including brachytherapy and external beam radiation therapy (EBRT). EBRT can be delivered as protons or photons, with photons being used in image-guided radiation therapy (IGRT). The fractionation schemes within these categories can vary, including conventional fractionation (~40 fractions), moderate hypofractionation (~20 fractions), or ultra-hypofractionation (~5 fractions), which is commonly referred to as stereotactic body radiation therapy (SBRT).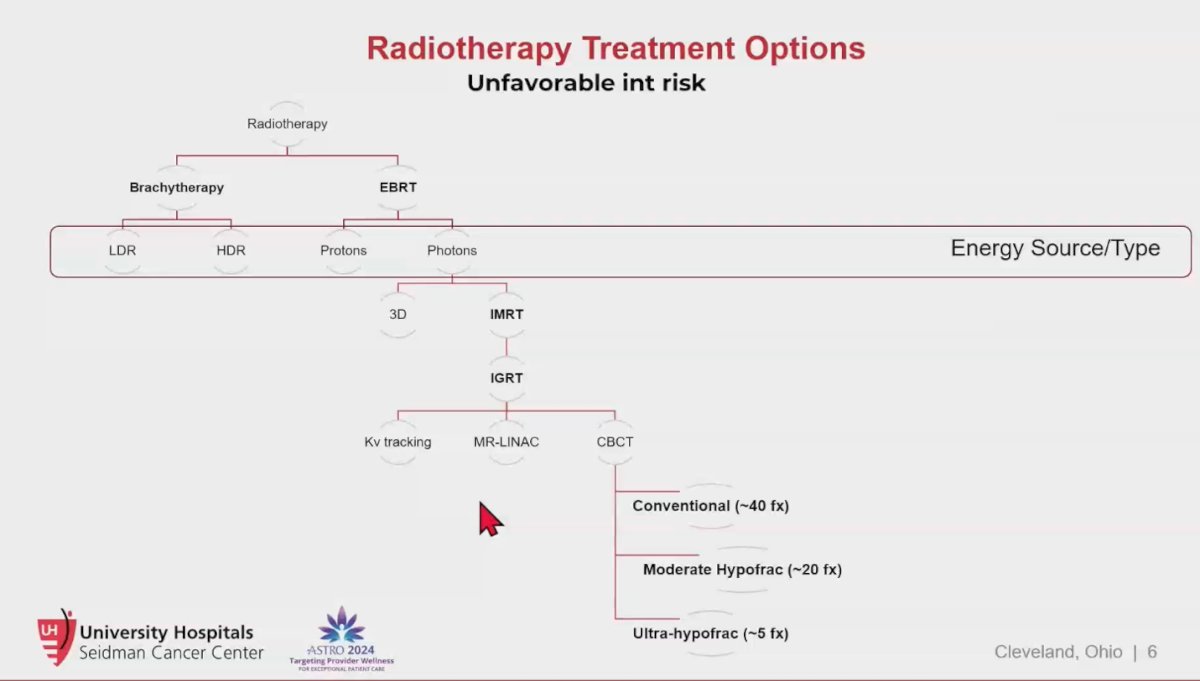
The evolution of radiotherapy treatment options for unfavorable intermediate-risk prostate cancer has been remarkable over the last 40 years. Starting in the 1980s with 2-dimensional radiotherapy, treatments were delivered in 45 fractions over 9 weeks, and there was a 10-30% risk of bothersome genitourinary and gastrointestinal toxicity, including a high risk of rectal bleeding. Today, we can treat patients with SBRT in just 5 fractions over 1-2 weeks, with only a 1-2% risk of bothersome toxicity.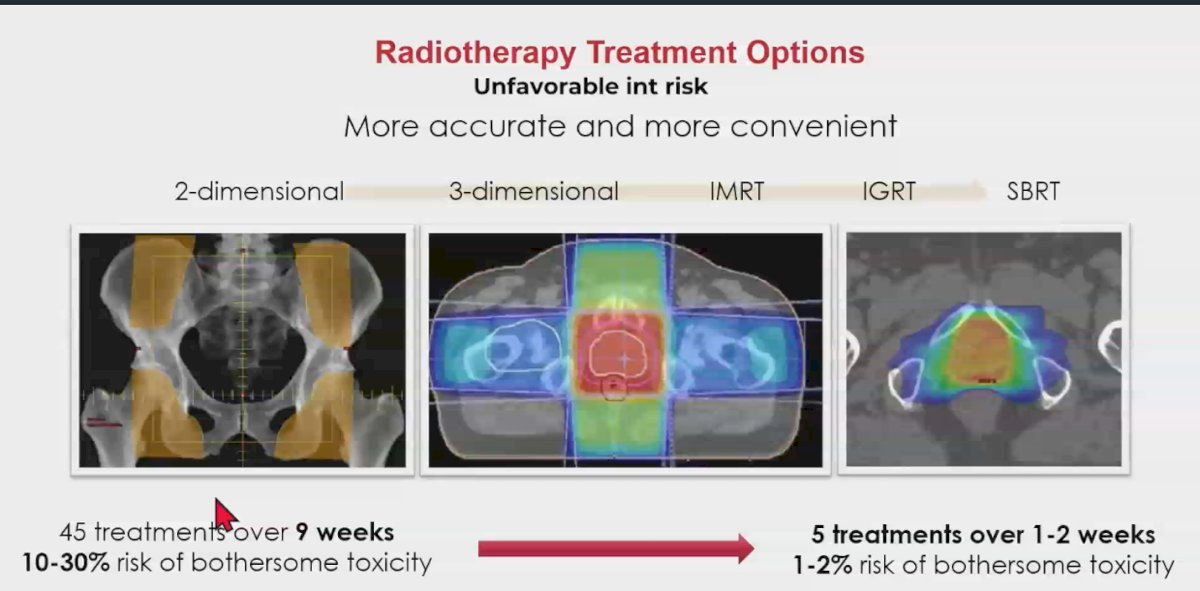
The NCCN guidelines recommend SBRT as a treatment option for nearly all risk categories, including unfavorable intermediate-risk prostate cancer. This recommendation is based on the HYPO-RT-PC phase 3 randomized non-inferiority trial involving 1,200 patients with intermediate and high-risk prostate cancer, which compared conventional fractionation (78Gy in 39 fractions of 2Gy) to ultra-hypofractionation (42.7Gy in 7 fractions of 6.1Gy). Notably, what is considered modern-day SBRT typically involves around 5 fractions, but this trial paved the way for further studies to explore and refine these approaches.2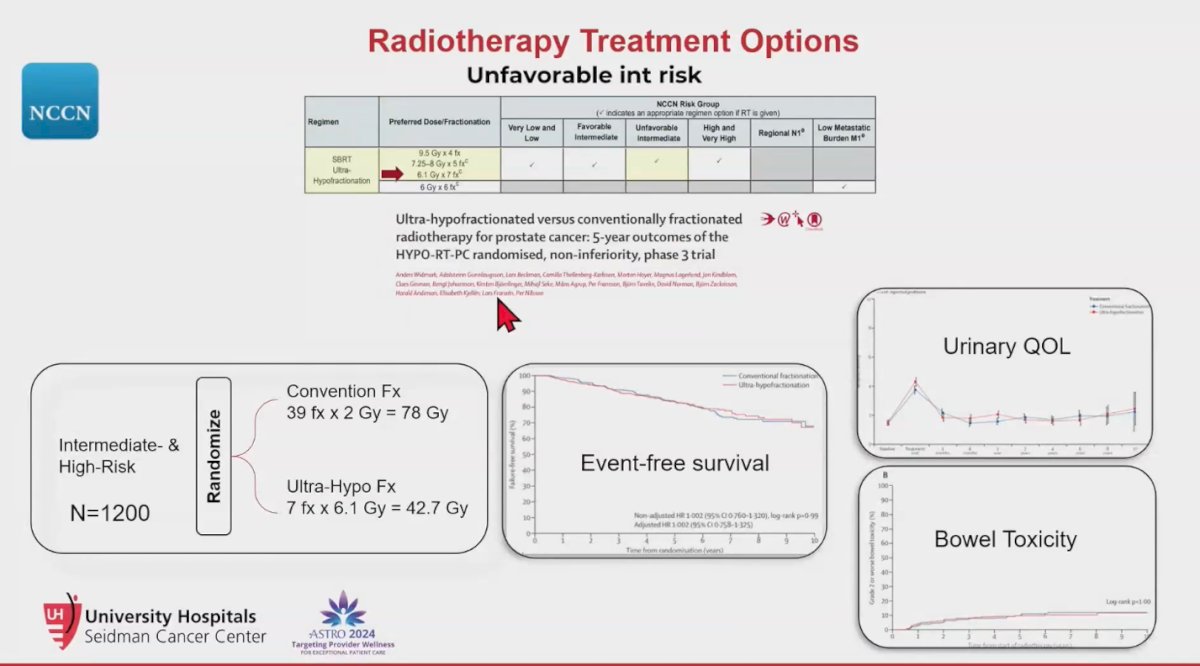
The PACE B trial 5-year outcomes, presented at the ASTRO meeting in 2023, was an international phase 3 randomized controlled trial comparing SBRT with conventionally fractionated or moderately hypofractionated RT in patients with low-risk (10%) and intermediate-risk (90%) prostate cancer. The SBRT trial scheme involved 5 fractions of 8Gy (CTV 40Gy) and 5 fractions of 7.25Gy (PTV 36.25Gy). The results showed no significant difference in event-free survival between the two groups, although there was a trend suggesting that SBRT might be slightly better. Urinary toxicity was higher in the SBRT group, with grade 2 or worse genitourinary events in 5.4% of SBRT patients compared to 3.7% in the conventional fractionation RT arm, although this difference was not significant (p=0.28). There were no significant differences in bowel toxicity. Overall, both treatment approaches exhibited very low grades of overall toxicity.3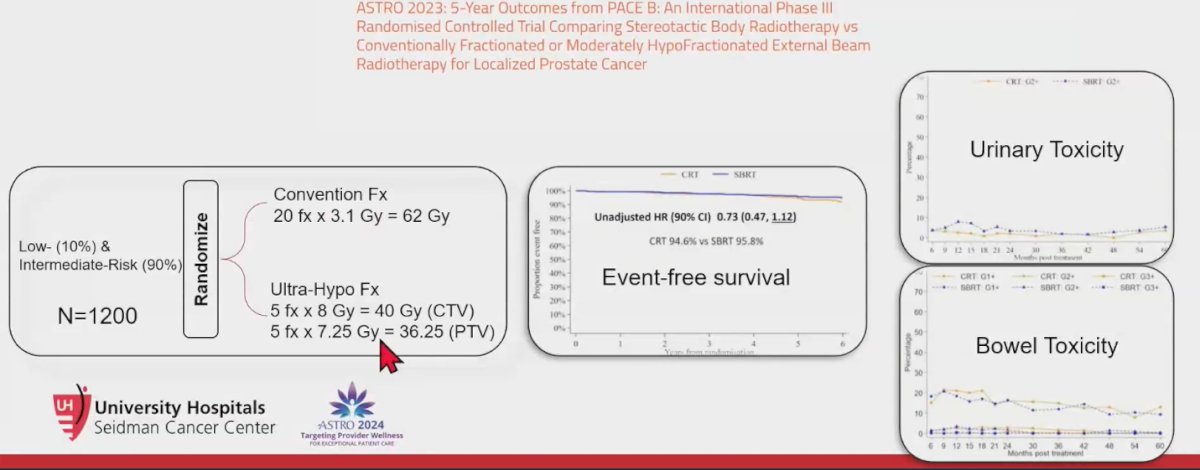
Notably, while the PACE B trial had an almost perfect trial design, a closer examination reveals some shortcomings. Specifically, the urethra contouring was not required, and many patients lacked MRI imaging to delineate the urethra, which was only constrained to "if visualized." The goal was to keep V44 Gy below 20% if the urethra was contoured. Consequently, it is likely that many patients received hotspots of 120% or greater, translating to doses of ≥ 48 Gy to the urethra, equivalent to approximately 121 Gy EQD2 (Equivalent Dose in 2 Gy fractions). This could explain the higher genitourinary toxicity observed in the trial.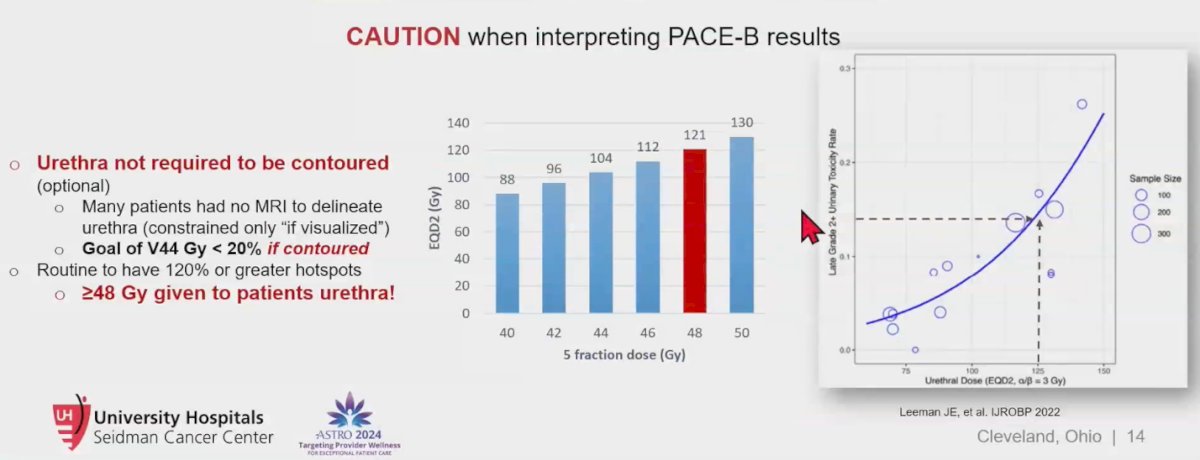
A systematic review and meta-analysis indicated excellent cancer control with stereotactic body radiation therapy (SBRT) but noted higher toxicity rates. The estimated rates of late grade ≥3 genitourinary (GU) and gastrointestinal (GI) toxicity were 2.0% (95% CI, 1.4%-2.8%) and 1.1% (95% CI, 0.6%-2.0%), respectively. Additionally, an increasing dose of SBRT was linked to improved biochemical control (P = .018) but also correlated with worse late grade ≥3 GU toxicity (P = .014). However, Dr. Spratt contends that SBRT is not inherently associated with higher toxicity, attributing the observed adverse effects primarily to a lack of dose constraints in the prospective series included in the meta-analysis.4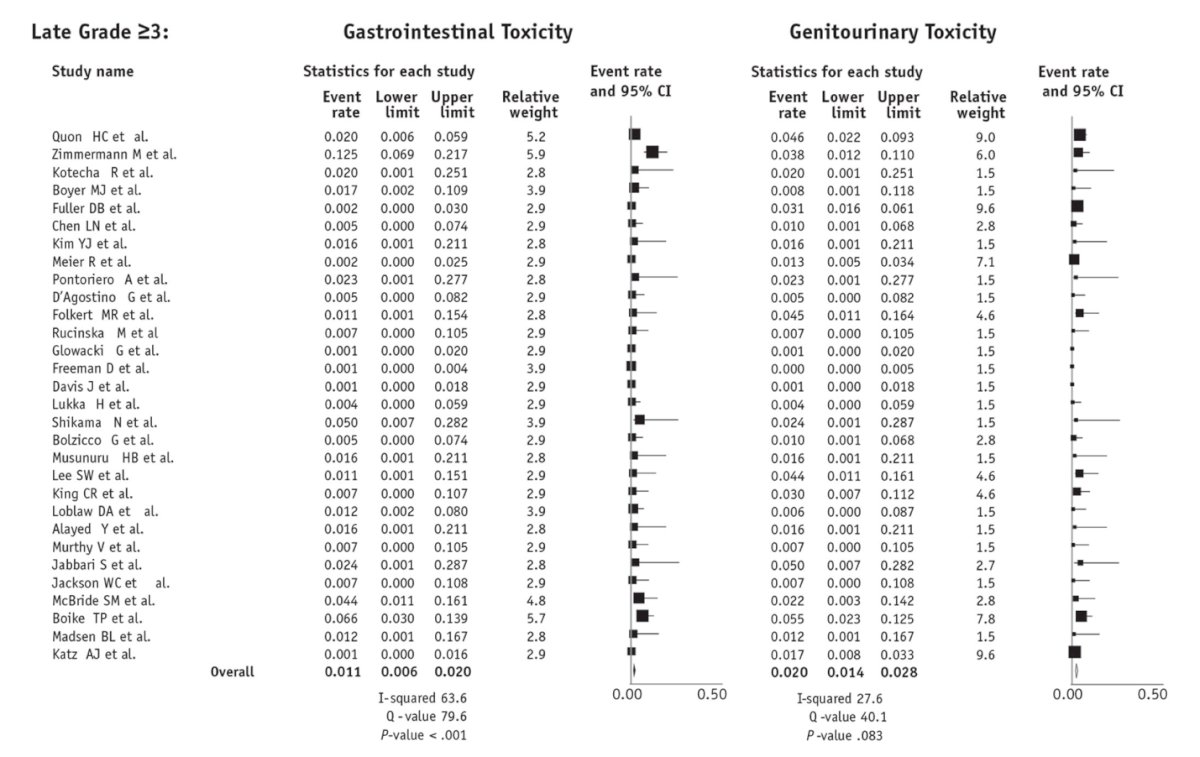
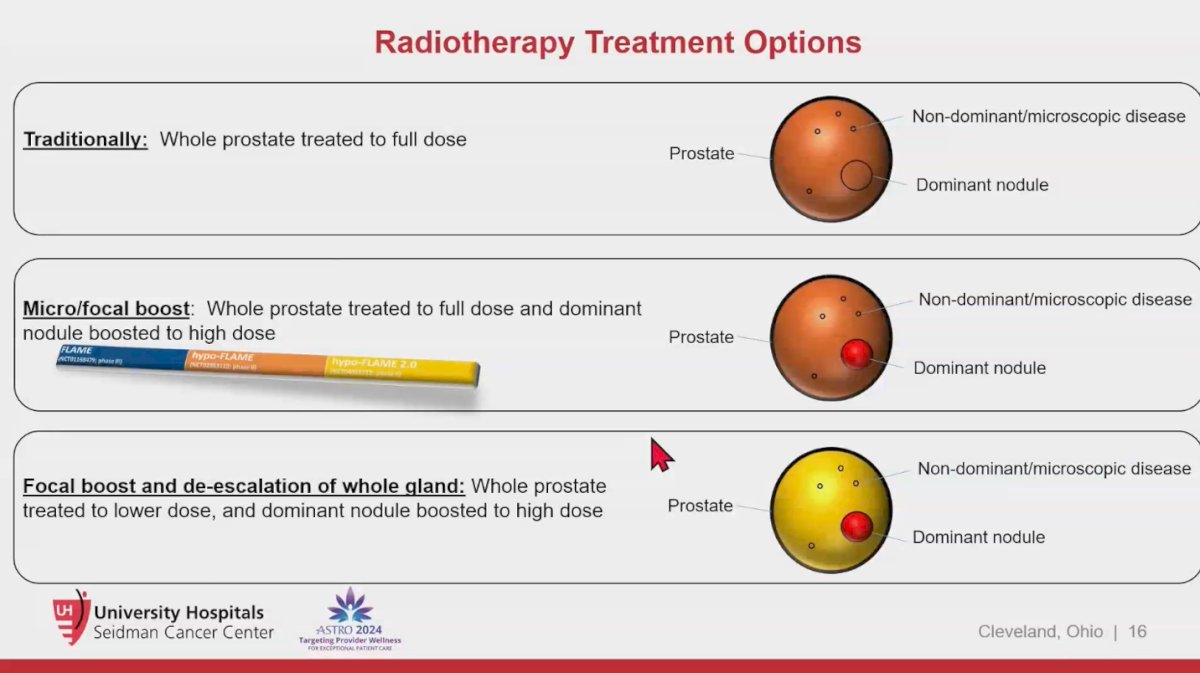
Dr. Spratt mentioned we could use SBRT in three different treatment modalities to treat prostate cancer:
- Traditional SBRT: Treat the whole prostate to the same dose
- Micro-focal boost: Whole prostate treated to full dose and dominant nodule boosted to high dose
- Focal boost and de-escalation of whole gland: Whole prostate treated to lower dose, and dominant nodule boosted to high dose (This is where the field is probably moving towards)
Micro-focal boost: Whole prostate treated to full dose and dominant nodule boosted to high dose
In a phase 1a/b trial evaluating 36.25 Gy delivered in 5 fractions to the prostate, a focal boost dose escalation was applied to the dominant intraprostatic nodule, with doses of 45 Gy, 47.5 Gy, and 50 Gy, all given in 5 fractions. The results indicated no grade 3 or higher toxicity reported, and the treatment was well tolerated among participants.5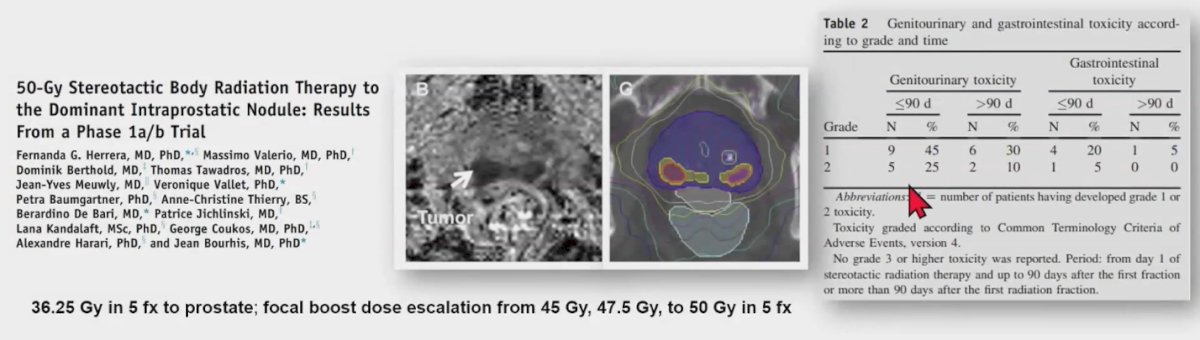
Focal boost and de-escalation of whole gland
Dr. Loblaws and colleagues assessed the feasibility of delivering a focal boost to the prostatic nodule while de-escalating the whole gland dose in patients with intermediate-risk and high-risk prostate cancer. The treatment regimen included a dose of 35 Gy to the prostate, 25 Gy to the pelvis, and a dose escalation to 50 Gy for the dominant intraprostatic lesion, all given in 5 fractions. The results indicated very good urinary quality of life and bowel quality of life. Additionally, acute grade ≥2 toxicity, late toxicity appeared to be comparable to a cohort that did not receive a focal boost.6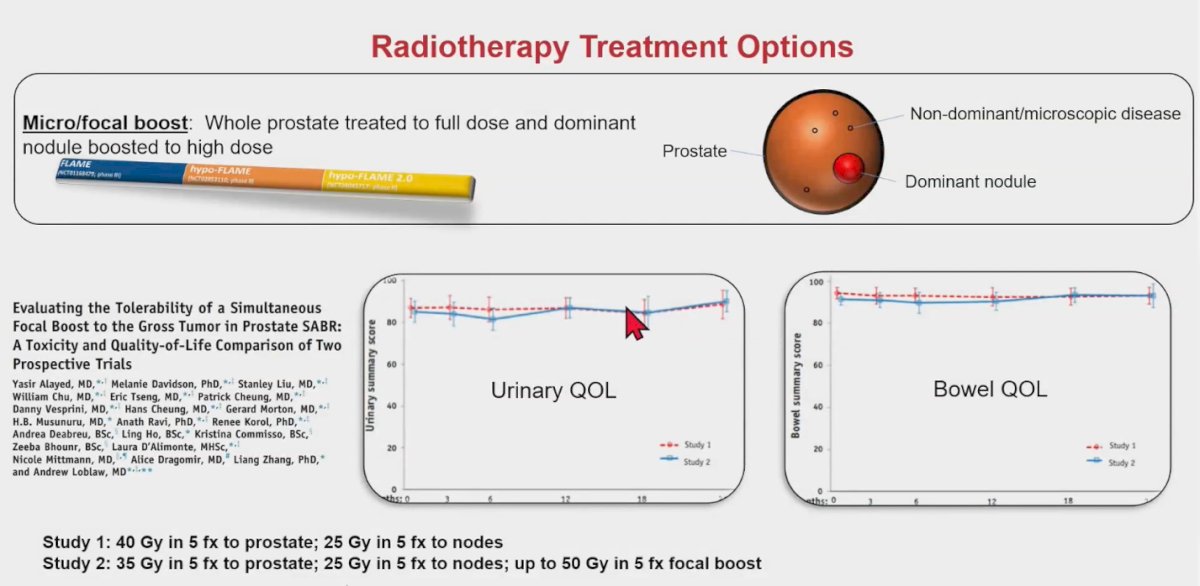
The FLAME 2.0 trial enrolled patients with intermediate- and high-risk prostate cancer who were treated with SBRT, delivering 35 Gy in 5 fractions to the whole prostate gland, with an iso-toxic boost of up to 50 Gy to the intraprostatic lesion using a semi-weekly (BIW) schedule compared to the prior weekly (QW) hypo-FLAME schedule. No grade ≥3 genitourinary (GU) or gastrointestinal (GI) toxicity was observed. Patients treated on the QW schedule experienced significantly less grade 2 GU toxicity (34.0%, p = 0.01). While this trial was randomized, it primarily aimed to compare different radiotherapy schedules.7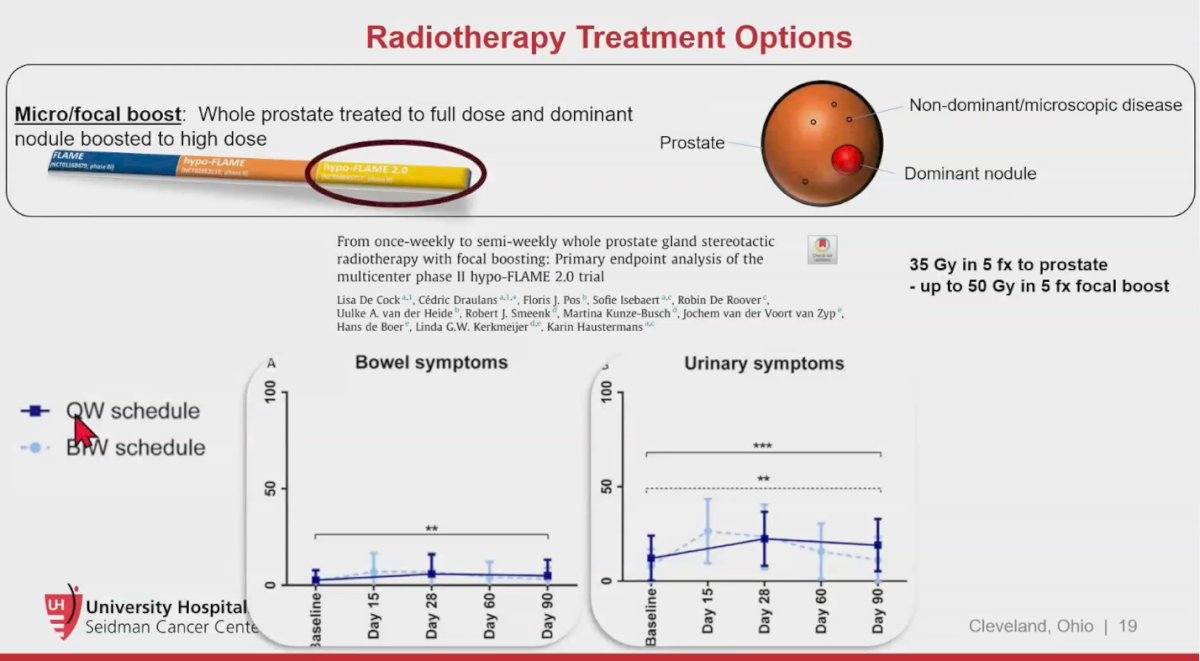
Dr. Spratt’s Protocol
Dr. Spratt presented a case of a patient with unfavorable intermediate-risk prostate cancer (cT1C, iPSA 6.1 ng/mL, GG3 with 4/13 cores). In his clinic, all patients with unfavorable intermediate-risk must ideally undergo an MRI prior to prostate fusion biopsy. They also receive a PSMA PET/CT scan and a 22-gene genomic classifier (Decipher) test. If they are participating in a trial, they additionally get MMAI as a predictive biomarker for ADT.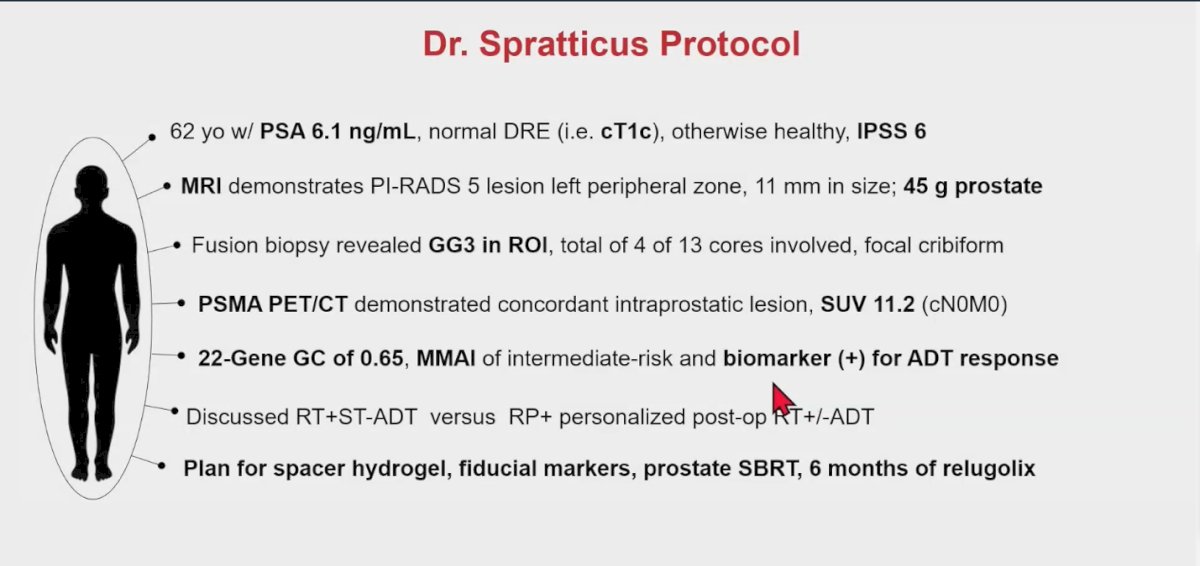
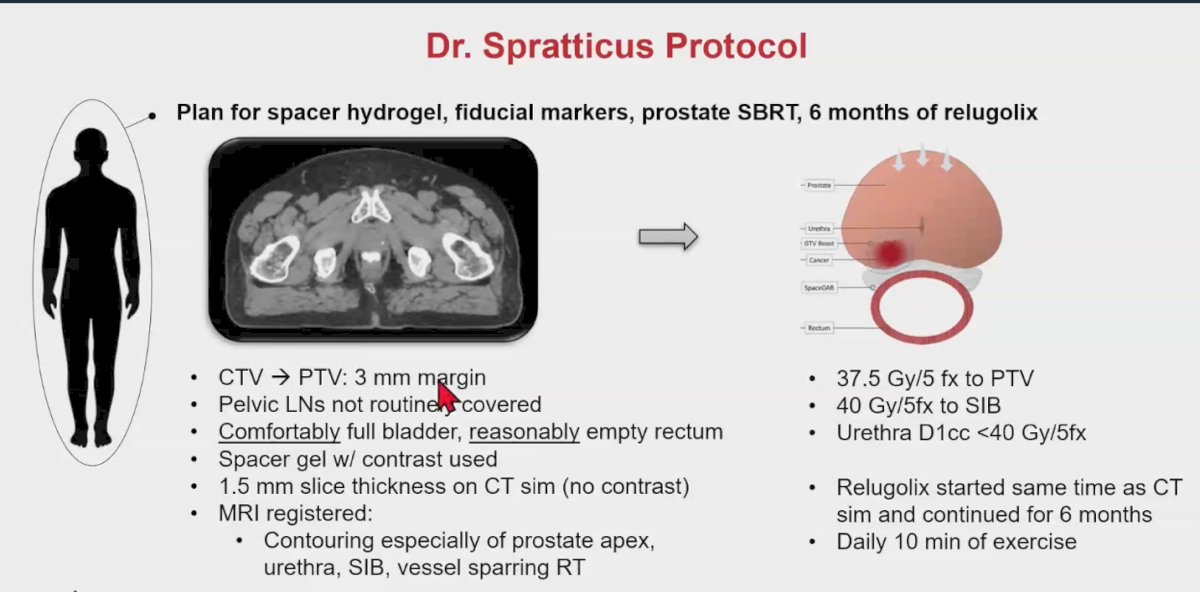
Their treatment protocol includes:
- CTV to PTV: 3mm Margin
- Pelvic lymph nodes are not routinely covered
- They use 7 mm margins into the rectum with no spacing
- The SBRT dose is 37.5 Gy/5 fraction to PTV and 40 Gy/5fraction to SIB
- The Urethra D1cc <40 Gy/5fraction
- He noted that he prefers to treat the dominant nodule to the whole dose (Full dose to the SIB)
Dr. Spratt conclude his presentation by discussing future directions in SBRT include the FORT trial, a randomized phase II study investigating MR-guided prostate stereotactic body radiotherapy (SBRT) administered in either 5 or 2 fractions for localized prostate cancer. The randomized HERMES trial, which compares 2 and 5-fraction magnetic resonance imaging-guided adaptive prostate radiation. Recently, an interim toxicity analysis from this trial reported acute grade 2 genitourinary (GU) toxicity in 1 (10%) patient in the 5-fraction group and 2 (20%) patients in the 2-fraction group, with no grade 3+ GU toxicities observed, recommending continuing recruitment to reach 23 participants per group.8 Additionally, the NRG-GU005 trial (NCI-2017-01398) is a phase III randomized study comparing SBRT (5 fractions of 7.25 Gy) to hypofractionated intensity-modulated radiation therapy (IMRT) (28 fractions of 2.5 Gy) in patients with intermediate-risk prostate cancer, with disease-free survival (DFS) as the primary outcome. Results from these studies are eagerly awaited.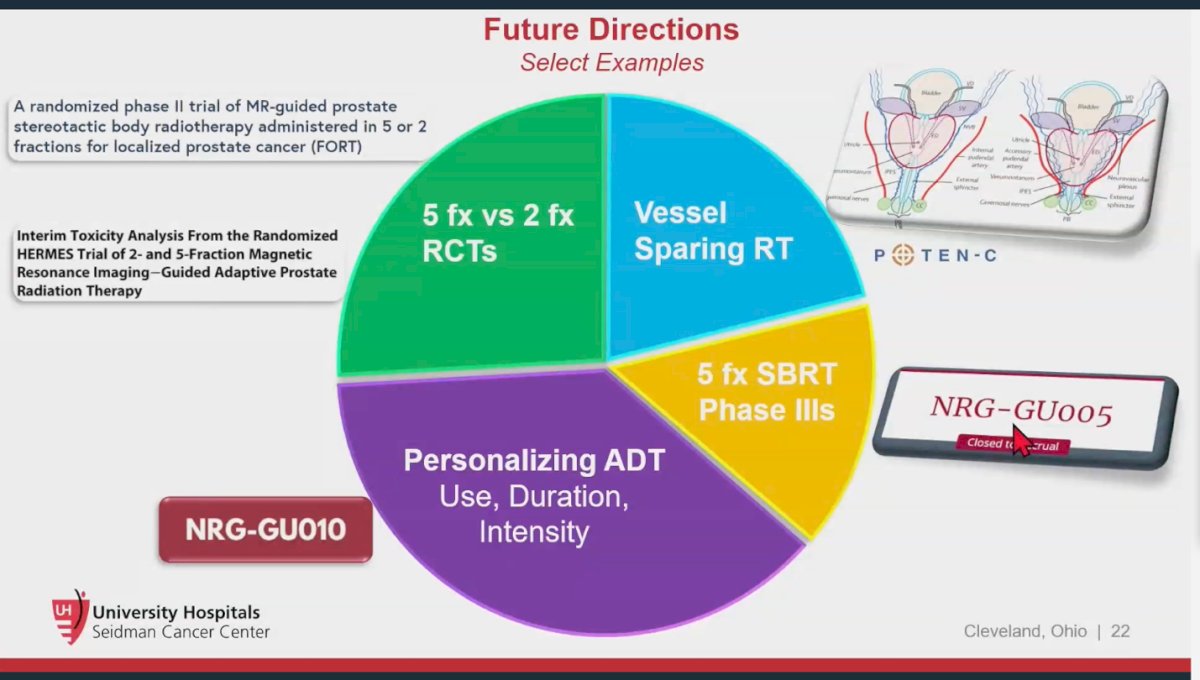
Presented by: Daniel Spratt, MD, Radiation Oncologist at University Hospitals Seidman Cancer Center, Beachwood, OH, United States of America
Written by: Julian Chavarriaga, MD – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @chavarriagaj on Twitter during the 2024 American Society for Radiation Oncology (ASTRO) annual meeting held in Washington D.C., between the 29th of September and the 2nd of October.
References:- Zumsteg ZS, Spratt DE, Daskivich TJ, Tighiouart M, Luu M, Rodgers JP, Sandler HM. Effect of Androgen Deprivation on Long-term Outcomes of Intermediate-Risk Prostate Cancer Stratified as Favorable or Unfavorable: A Secondary Analysis of the RTOG 9408 Randomized Clinical Trial. JAMA Netw Open. 2020 Sep 1;3(9):e2015083. doi: 10.1001/jamanetworkopen.2020.15083. PMID: 32902647; PMCID: PMC7489808.
- Widmark A, Gunnlaugsson A, Beckman L, Thellenberg-Karlsson C, Hoyer M, Lagerlund M, Kindblom J, Ginman C, Johansson B, Björnlinger K, Seke M, Agrup M, Fransson P, Tavelin B, Norman D, Zackrisson B, Anderson H, Kjellén E, Franzén L, Nilsson P. Ultra-hypofractionated versus conventionally fractionated radiotherapy for prostate cancer: 5-year outcomes of the HYPO-RT-PC randomised, non-inferiority, phase 3 trial. Lancet. 2019 Aug 3;394(10196):385-395. doi: 10.1016/S0140-6736(19)31131-6. Epub 2019 Jun 18. PMID: 31227373.
- van As, N. et al. 5-Year Outcomes from PACE B: An International Phase III Randomized Controlled Trial Comparing Stereotactic Body Radiotherapy (SBRT) vs. Conventionally Fractionated or Moderately Hypo Fractionated External Beam Radiotherapy for Localized Prostate Cancer. International Journal of Radiation Oncology, Biology, Physics, Volume 117, Issue 4, e2 - e3
- Jackson WC, Silva J, Hartman HE, Dess RT, Kishan AU, Beeler WH, Gharzai LA, Jaworski EM, Mehra R, Hearn JWD, Morgan TM, Salami SS, Cooperberg MR, Mahal BA, Soni PD, Kaffenberger S, Nguyen PL, Desai N, Feng FY, Zumsteg ZS, Spratt DE. Stereotactic Body Radiation Therapy for Localized Prostate Cancer: A Systematic Review and Meta-Analysis of Over 6,000 Patients Treated On Prospective Studies. Int J Radiat Oncol Biol Phys. 2019 Jul 15;104(4):778-789. doi: 10.1016/j.ijrobp.2019.03.051. Epub 2019 Apr 6. PMID: 30959121; PMCID: PMC6770993.
- Herrera FG, Valerio M, Berthold D, Tawadros T, Meuwly JY, Vallet V, Baumgartner P, Thierry AC, De Bari B, Jichlinski P, Kandalaft L, Coukos G, Harari A, Bourhis J. 50-Gy Stereotactic Body Radiation Therapy to the Dominant Intraprostatic Nodule: Results From a Phase 1a/b Trial. Int J Radiat Oncol Biol Phys. 2019 Feb 1;103(2):320-334. doi: 10.1016/j.ijrobp.2018.09.023. Epub 2018 Sep 27. PMID: 30267761.
- Alayed Y, Davidson M, Liu S, Chu W, Tseng E, Cheung P, Vesprini D, Cheung H, Morton G, Musunuru HB, Ravi A, Korol R, Deabreu A, Ho L, Commisso K, Bhounr Z, D'Alimonte L, Mittmann N, Dragomir A, Zhang L, Loblaw A. Evaluating the Tolerability of a Simultaneous Focal Boost to the Gross Tumor in Prostate SABR: A Toxicity and Quality-of-Life Comparison of Two Prospective Trials. Int J Radiat Oncol Biol Phys. 2020 May 1;107(1):136-142. doi: 10.1016/j.ijrobp.2019.12.044. Epub 2020 Jan 25. PMID: 31987962.
- De Cock L, Draulans C, Pos FJ, Isebaert S, De Roover R, van der Heide UA, Smeenk RJ, Kunze-Busch M, van der Voort van Zyp J, de Boer H, Kerkmeijer LGW, Haustermans K. From once-weekly to semi-weekly whole prostate gland stereotactic radiotherapy with focal boosting: Primary endpoint analysis of the multicenter phase II hypo-FLAME 2.0 trial. Radiother Oncol. 2023 Aug;185:109713. doi: 10.1016/j.radonc.2023.109713. Epub 2023 May 11. PMID: 37178932.
- Westley RL, Biscombe K, Dunlop A, Mitchell A, Oelfke U, Nill S, Murray J, Pathmanathan A, Hafeez S, Parker C, Ratnakumaran R, Alexander S, Herbert T, Hall E, Tree AC. Interim Toxicity Analysis From the Randomized HERMES Trial of 2- and 5-Fraction Magnetic Resonance Imaging-Guided Adaptive Prostate Radiation Therapy. Int J Radiat Oncol Biol Phys. 2024 Mar 1;118(3):682-687. doi: 10.1016/j.ijrobp.2023.09.032. Epub 2023 Sep 29. PMID: 37776979.


