(UroToday.com) The 2024 American Society for Radiation Oncology (ASTRO) Annual Meeting held in Washington, DC was host to a prostate cancer treatment intensification session. Dr. Colin Belliveau presented the results of a randomized controlled trial of PSMA PET image-guided intensification of salvage radiotherapy after a radical prostatectomy.
It has been demonstrated that 18F-DCFPyL PSMA PET/CT improves prostate cancer detection and can alter the management of a significant proportion of patients.1-3 However, to date, it remains unknown whether PSMA-PET guided intensification of radiotherapy treatment plans improves failure-free survival.
This trial (NCT03525288) included 128 patients receiving post-radical prostatectomy salvage therapy (n=128). Patients were eligible if they experienced biochemical failure post-radical prostatectomy (PSA≥0.1 ng/ml) and were planned for salvage radiotherapy. They were ineligible if they had received ADT within 12 months and had a prior PSMA PET performed.
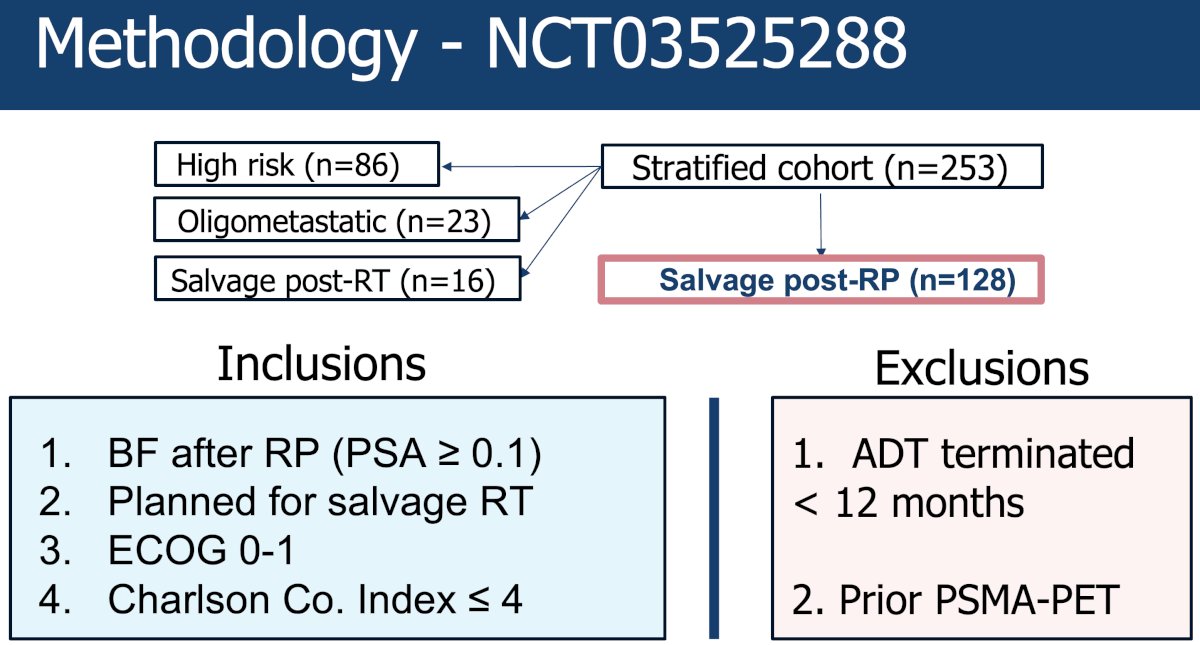
Eligible patients were randomized 1:1 to either standard of care salvage radiotherapy (prostate bed +/- pelvis) versus PSMA PET-intensified salvage radiotherapy. An example of a PSMA PET-guided intensification approach is illustrated below:

The baseline patient characteristics are summarized below. Overall, patients in the experimental arm had worse risk disease features. Grade Group 5 disease was present more commonly in the experimental arm (22% versus 5%, p=0.01). There was a higher surgical margin positivity rate in the experimental arm, as well (53% versus 32%, p=0.02).

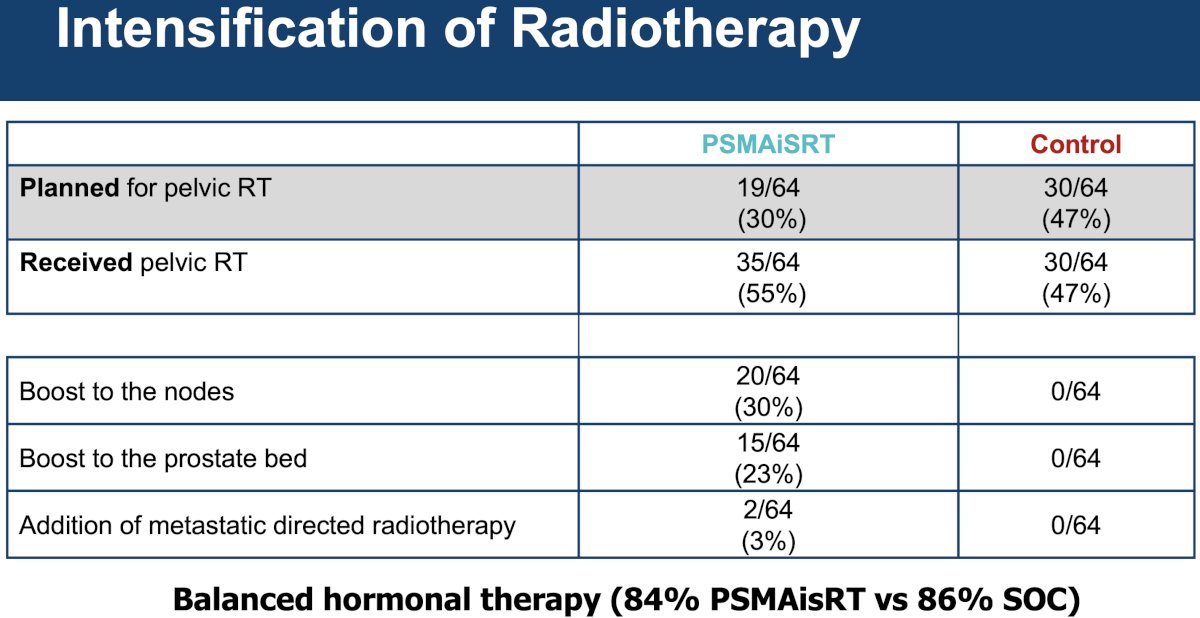
The radiotherapy intensification results are summarized in the table below. In the PSMA PET-guided intensification arm, 30% of patients received a nodal boost and 23% a prostatic bed boost. 3% of patients received additional metastasis-directed radiotherapy.
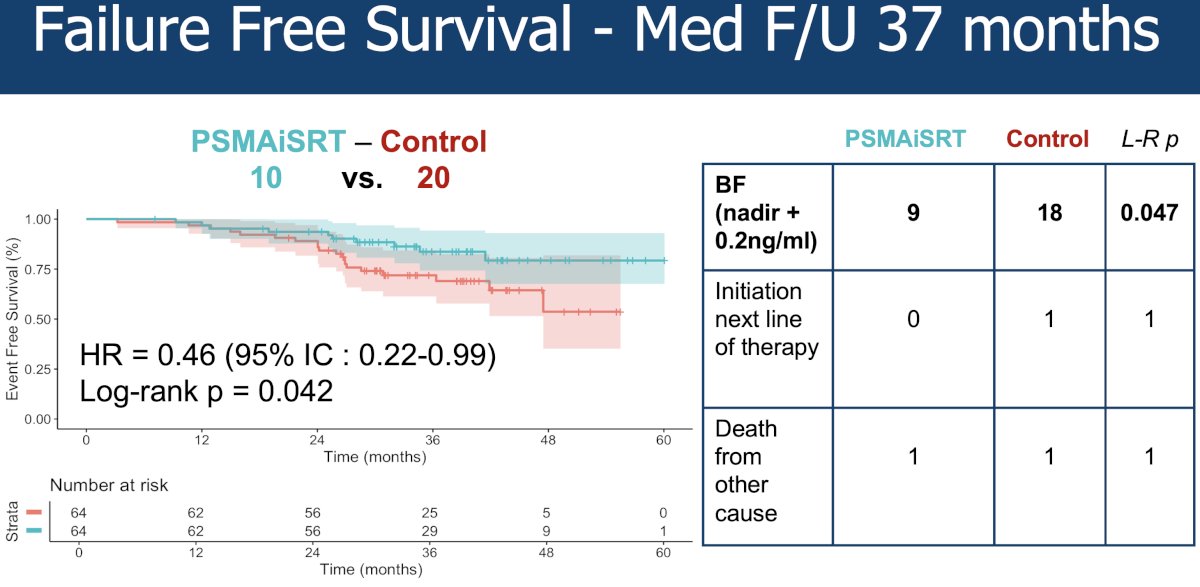
At a median follow-up of 37 months, patients in the PSMA PET-guided intensification arm had superior failure-free survival outcomes (HR; 0.46, 95% CI: 0.22–0.99, p=0.042).
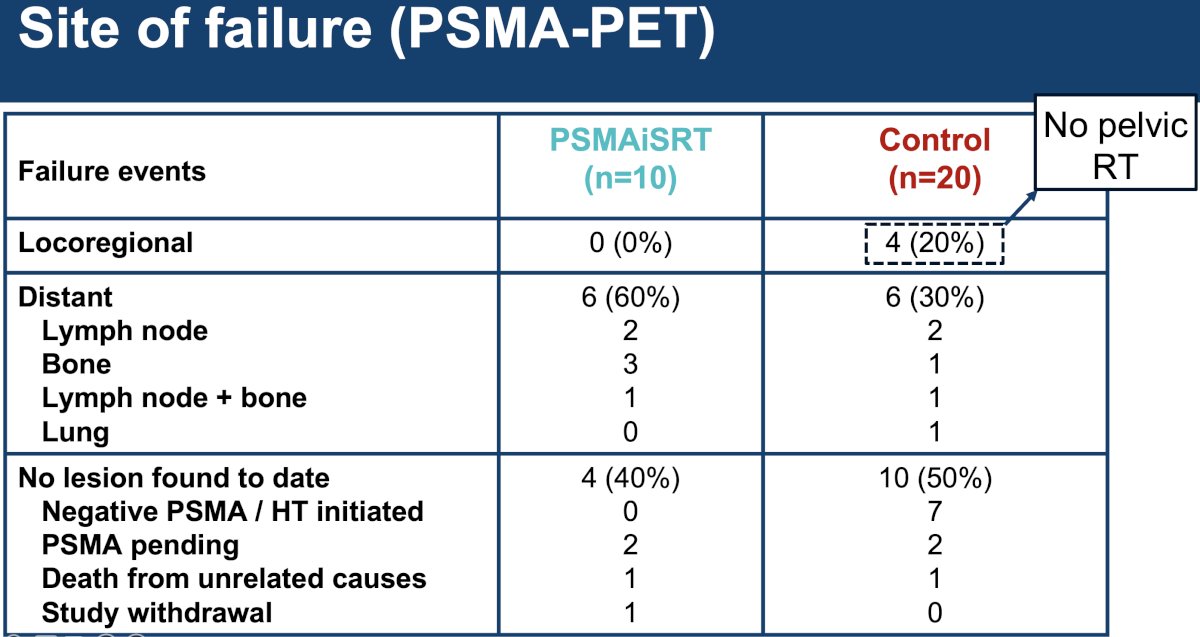
The sites of failure are summarized below. Notably, none of the 4 patients in the control arm who experienced locoregional failure had received pelvic radiotherapy.
Patients in the experimental arm also had longer treatment-free survivals (i.e., next line hormone therapy +/- metastasis-directed radiotherapy; HR: 0.32, 95% CI: 0.1–1.0, p=0.038).

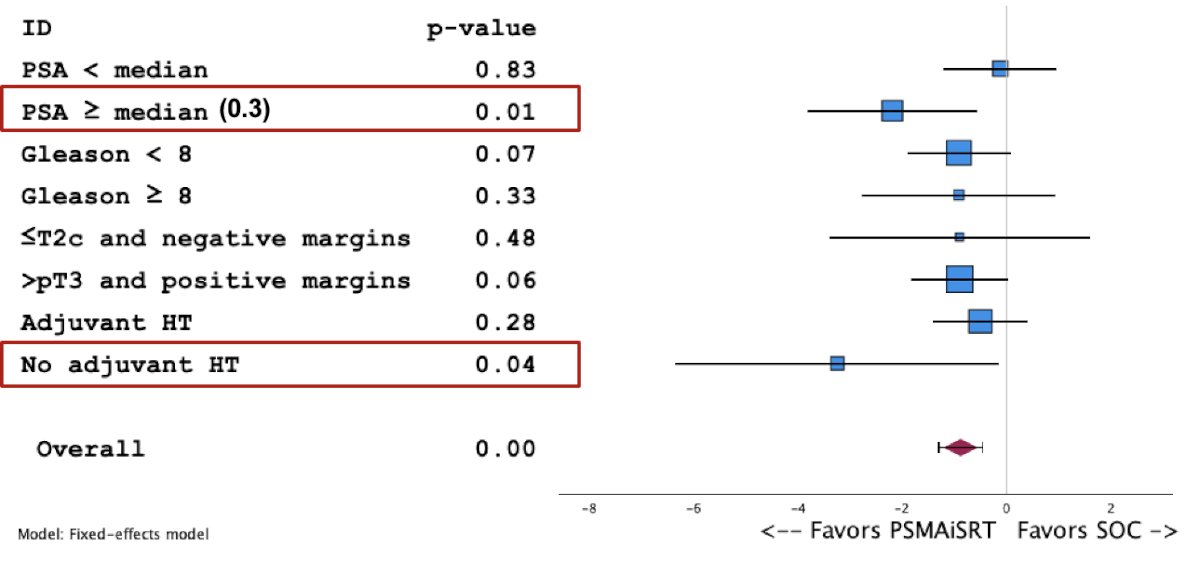
On subgroup analysis, there was a significant benefit in favor of PSMA-guided intensification of salvage therapy in those who had a pre-treatment PSA level ≥0.3 ng/ml (p=0.01) and in those who had not received adjuvant hormone therapy (p=0.04).
There were no differences in the rates of time-to-first Grade ≥2 adverse events:
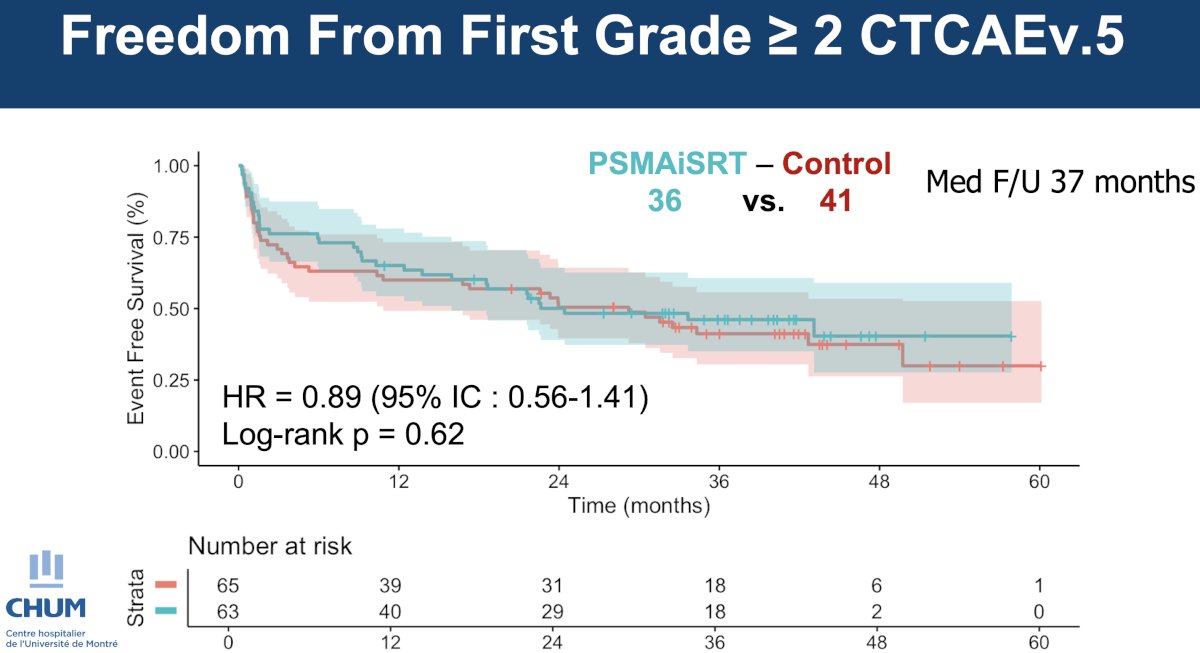
Overall, there were no differences in the proportions of the individual adverse events. One patient had grade 3 events attributable to intensified radiotherapy (GU stenosis requiring surgical intervention).

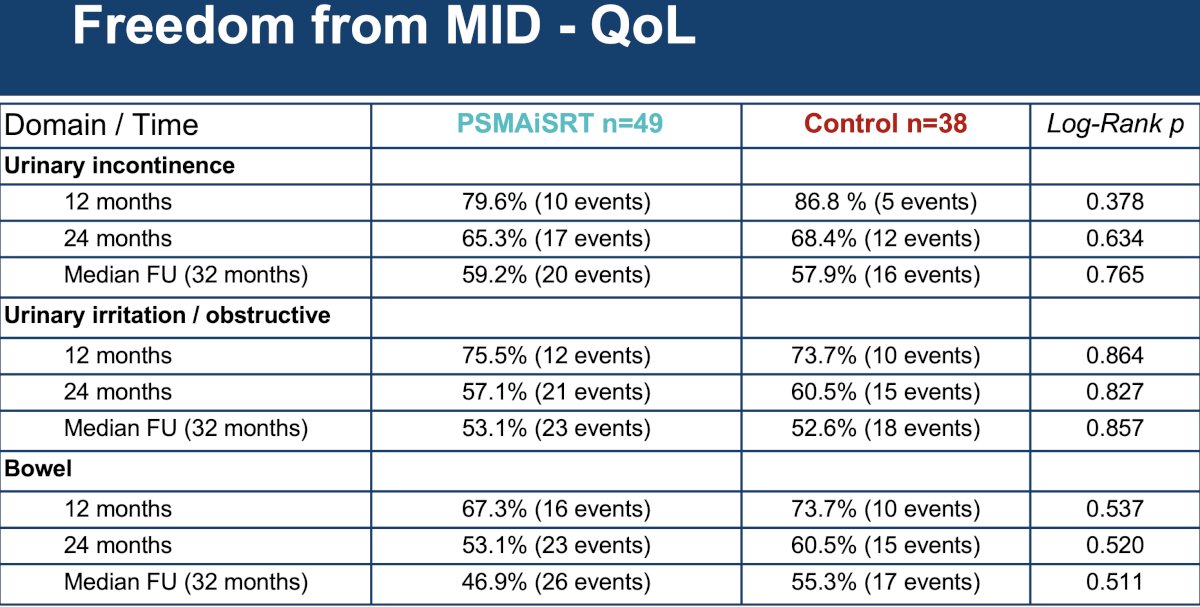
With regards to quality of life, patients in the intensified treatment arm had superior sexual domain scores at both 12- and 24-months follow-up. Dr. Belliveau noted that this may be possibly related to the administration of second-line hormone therapy at biochemical failure.

Dr. Belliveau concluded as follows:
- This randomized trial demonstrated ‘isotoxic’ improvements in failure-free and treatment-free survivals with PSMA PET-intensified salvage radiotherapy, particularly in patients with a PSA ≥0.3 ng/ml.
- A confirmatory phase III randomized trial (PATRON; NCT04557501) has completed accrual.
Presented by: Colin Belliveau, MD, Resident Physician, Département de radio-oncologie, Centre Hospitalier de l'Université de Montréal (CHUM), Montréal, Québec, Canada.
Written by: Rashid Sayyid, MD, MSc – Robotic Urologic Oncology Fellow at The University of Southern California, @rksayyid on Twitter during the 2024 ASTRO Annual Congress held in Washington, DC between September 29th and October 2nd, 2024
References:
- Calais J, Ceci F, Eiber M, et al. 18F-fluciclovine PET-CT and 68Ga-PSMA-11 PET-CT in patients with early biochemical recurrence after prostatectomy: a prospective, single-centre, single-arm, comparative imaging trial. Lancet Oncol. 2019;20(9):1286-94.
- Fendler WP, Calais J, Eiber M, et al. Assessment of 68Ga-PSMA-11 PET Accuracy in Localizing Recurrent Prostate Cancer. JAMA Oncol. 2019; 5(6):856-63.
- Morris MJ, Rowe SP, Gorin MA, et al. Diagnostic Performance of 18F-DCFPyL-PET/CT in Men with Biochemically Recurrent Prostate Cancer: Results from the CONDOR Phase 3, Multicenter Study. Clin Cancer Res. 2021;27(13):3674-82.


