(UroToday.com) The 2024 American Society for Radiation Oncology (ASTRO) Annual Meeting held in Washington, DC was host to a prostate cancer treatment intensification session. Dr. Karen Hoffman presented the long-term patient-reported health-related quality of life (HRQoL) outcomes from the FORMULA-509 randomized trial of salvage radiotherapy and 6 months of a GnRH agonist plus either bicalutamide or abiraterone plus prednisone and apalutamide following a radical prostatectomy.
FORMULA-509 is an investigator-initiated, multicenter, open-label, randomized phase II trial that included patients with a PSA ≥0.1 ng/ml post-radical prostatectomy and who had ≥1 unfavorable risk feature (Gleason Score 8–10, PSA>0.5 ng/ml, pT3-4, pN1 or cN1, PSA doubling time <10 months, negative margins, persistent PSA, gross local/regional disease, or Decipher High Risk). All eligible patients received salvage radiotherapy plus 6 months of a GnRH agonist and randomization was to concurrent bicalutamide 50 mg or abiraterone acetate/prednisone 1000 mg/5 mg +apalutamide 240 mg daily. Radiation to the pelvic nodes was required for pN1 and optional for pN0.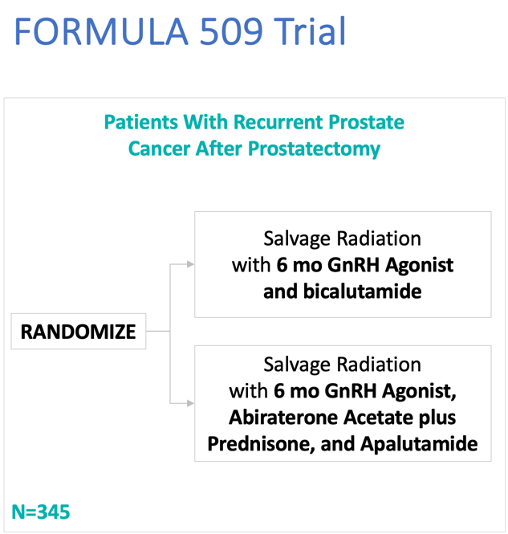
Previously published efficacy results of this trial have demonstrated that the addition of abiraterone acetate/prednisone and apalutamide improved metastasis-free survival, compared to bicalutamide (HR: 0.32, 95% CI: 0.13– 0.84, p=0.02):1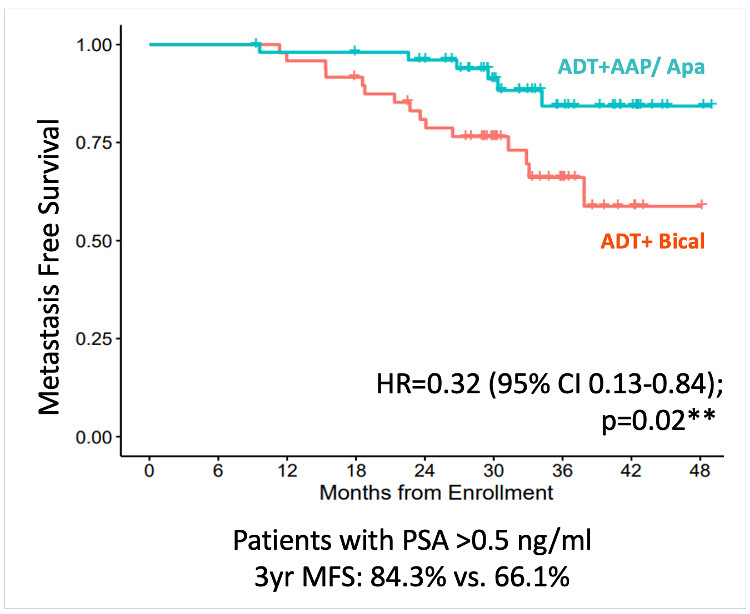
Among patients randomized to the more intense androgen axis regimen, physician-reported adverse events were consistent with the known safety profile of abiraterone acetate/prednisone and apalutamide: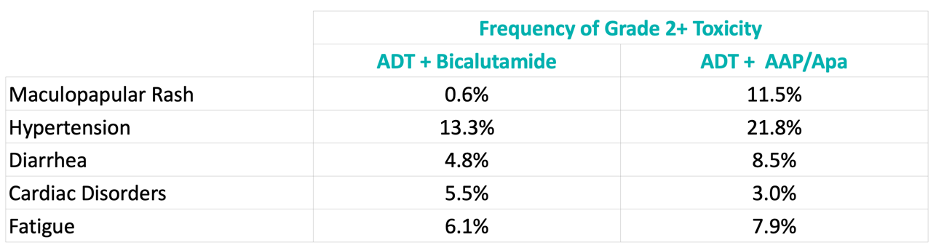
However, Dr. Hoffman noted that it is critical to understand the impact on long-term patient reported HRQoL measures, with previous reports from this trial only describing the 1-year outcomes. In this presentation, Dr. Hoffman presented the longer-term 3-year outcomes.
Validated questionnaires were administered at baseline, end of treatment, and one-, and three-years following completion of treatment.
The questionnaire completion rates were excellent at baseline (95–96%). This decreased to ~80% at end of treatment, ~70% at one year follow-up, and down to 57% (EPIC-26 and PROMIS Fatigue Short Form 4) and 34% (SLUMS) at three-years post-treatment.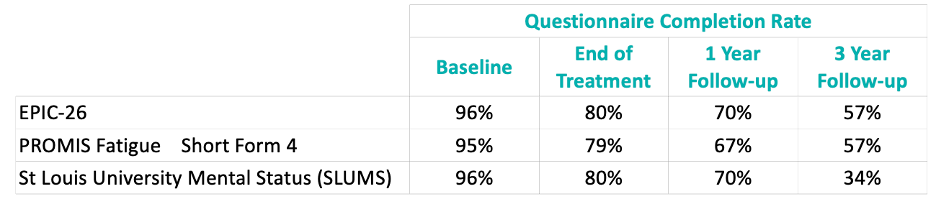
The baseline patient characteristics are summarized below. The median patient age was 64 years. 35% had Gleason 9 disease. The median serum PSA at randomization was 0.3 ng/ml. 74% of patients received pelvic nodal treatment.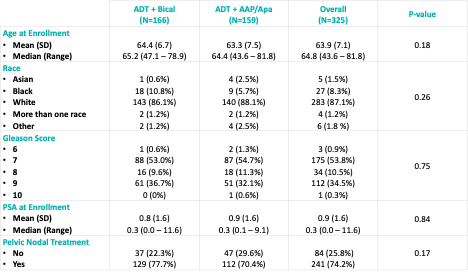
There were no differences in patient-reported EPIC-26 hormonal function scores between the treatment arms at the end of treatment, 1-, and 3 years post-treatment completion.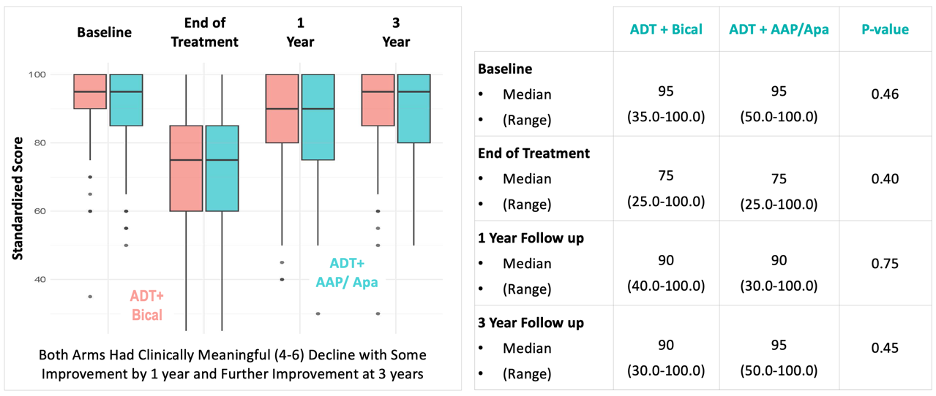
Similarly, there were no differences in the proportion of patients with moderate or big problems in the EPIC-26 individual hormonal questions between treatment arms at the end of treatment and at both 1- and 3-years post-treatment.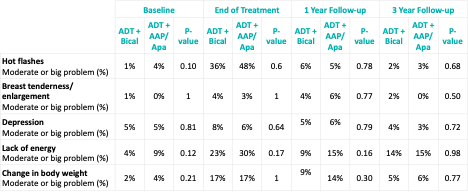
There were no differences in patient-reported EPIC-26 sexual function domain scores between the treatment arms at all assessed time points.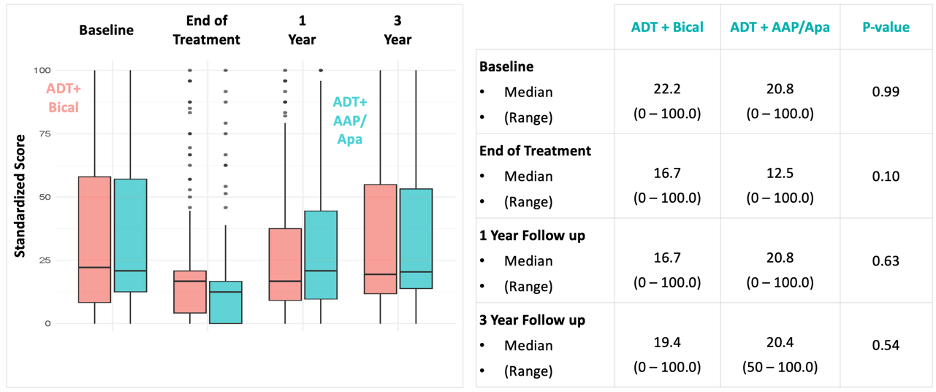
Patient-reported fatigue was similar for both treatment arms across all assessed time points.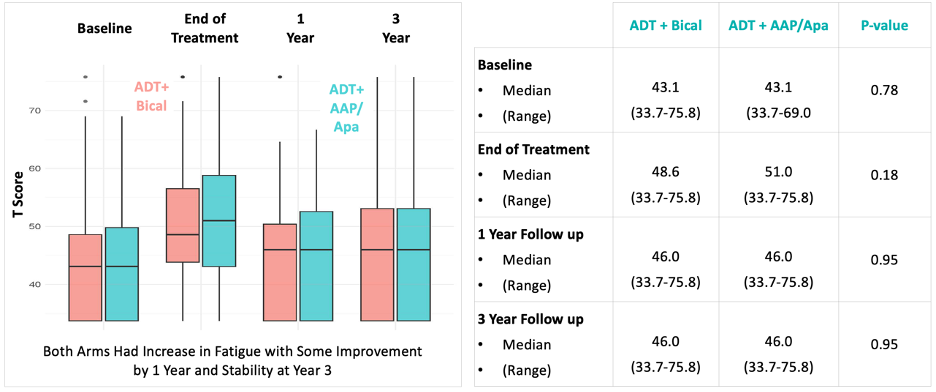
There were no differences in cognitive impairment scores, assessed using the SLU Mental Status Exam, between treatment arms at the end of treatment and one-year post-treatment.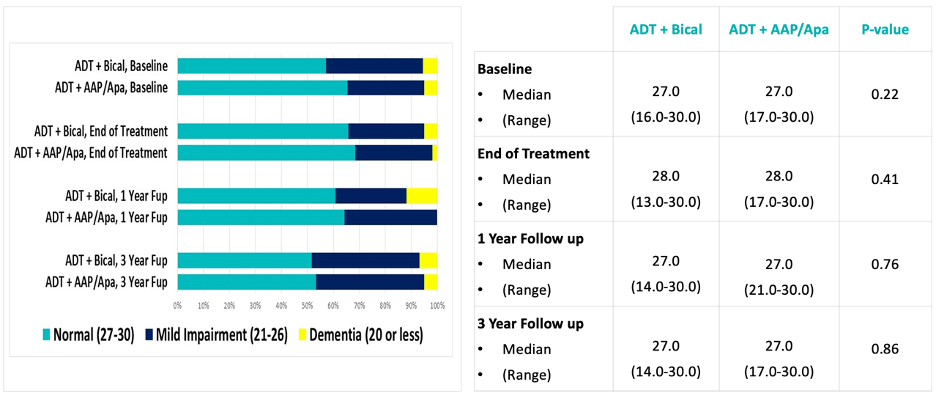
How do these results compare to those from the RADICALS-HD trial? While RADICALS-HD demonstrated that 24 months of ADT also improved metastasis-free survival, compared to 6 months of ADT,2 in the FORMULA 509 trial, 86% of patients recovered eugonadal testosterone levels by 24 months (RADICALS-HD: 78%), which suggests potential superior HRQoL outcomes with 6 months of intensified abiraterone + apalutamide, compared to 24 months of ADT.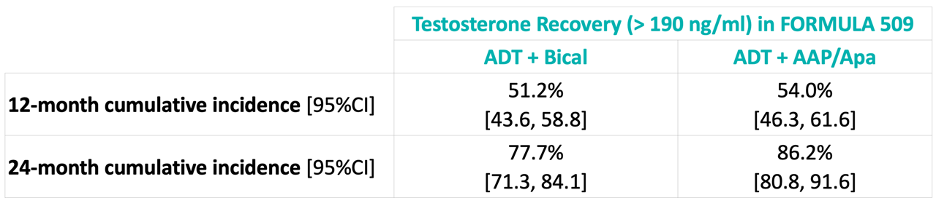
Dr. Hoffman concluded as follows:
- In the Formula 509 randomized trial, the addition of abiraterone acetate/prednisone + apalutamide to salvage radiotherapy with 6 months of ADT improved oncologic outcomes, without causing a detectable difference in patient-reported hormonal function, sexual function, fatigue, or mental status through three years after treatment, compared to bicalutamide
- Given the favorable short- and long-term patient-reported health-related quality of life outcomes, 6 months of intensified ADT with next generation anti-androgens is an attractive treatment alternative to long-duration ADT for patients with rising PSA and unfavorable features after radical prostatectomy
Presented by: Karen Hoffman, MD, MHSc, MPH, FASTRO, Professor, Department of GU Radiation Oncology, Division of Radiation Oncology, The University of Texas MD Anderson Cancer Center, Houston, TX
Written by: Rashid Sayyid, MD, MSc – Robotic Urologic Oncology Fellow at The University of Southern California, @rksayyid on Twitter during the 2024 ASTRO Annual Congress held in Washington, DC between September 29th and October 2nd, 2024
References:
- Nguyen PL, Kollmeier MA, Rathkopf D, et al. FORMULA-509: A Multicenter Randomized Trial of Post-Operative Salvage Radiotherapy (SRT) and 6 Months of GnRH Agonist with Either Bicalutamide or Abiraterone Acetate/Prednisone (AAP) and Apalutamide (Apa) Post-Radical Prostatectomy (RP). Int J Radiat Oncol Biol Phys. 2023; 117(2):S81-2.


